Targeted deletion of Fgf9 in tendon disrupts mineralization of the developing enthesis
- PMID: 36734881
- PMCID: PMC10108073
- DOI: 10.1096/fj.202201614R
Targeted deletion of Fgf9 in tendon disrupts mineralization of the developing enthesis
Erratum in
-
Erratum to "Targeted deletion of Fgf9 in tendon disrupts mineralization of the developing enthesis".FASEB J. 2023 Apr;37(4):e22856. doi: 10.1096/fsb2.22856. FASEB J. 2023. PMID: 36929525 Free PMC article. No abstract available.
Abstract
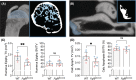
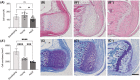
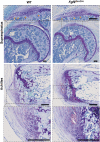
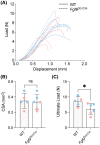
The enthesis is a transitional tissue between tendon and bone that matures postnatally. The development and maturation of the enthesis involve cellular processes likened to an arrested growth plate. In this study, we explored the role of fibroblast growth factor 9 (Fgf9), a known regulator of chondrogenesis and vascularization during bone development, on the structure and function of the postnatal enthesis. First, we confirmed spatial expression of Fgf9 in the tendon and enthesis using in situ hybridization. We then used Cre-lox recombinase to conditionally knockout Fgf9 in mouse tendon and enthesis (Scx-Cre) and characterized enthesis morphology as well as mechanical properties in Fgf9ScxCre and wild-type (WT) entheses. Fgf9ScxCre mice had smaller calcaneal and humeral apophyses, thinner cortical bone at the attachment, increased cellularity, and reduced failure load in mature entheses compared to WT littermates. During postnatal development, we found reduced chondrocyte hypertrophy and disrupted type X collagen (Col X) in Fgf9ScxCre entheses. These findings support that tendon-derived Fgf9 is important for functional development of the enthesis, including its postnatal mineralization. Our findings suggest the potential role of FGF signaling during enthesis development.
Keywords: attachment; enthesis; fibroblast growth factor; musculoskeletal; postnatal; tendon.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures



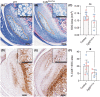
References
-
- Galatz L, Rothermich S, VanderPloeg K, Petersen B, Sandell L, Thomopoulos S. Development of the supraspinatus tendon‐to‐bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res. 2007;25(12):1621‐1628. - PubMed
-
- Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon‐bone attachment unit is formed modularly by a distinct pool of Scx ‐ and Sox9 ‐positive progenitors. Development. 2013;140(13):2680‐2690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

