Immunogenicity of a Fourth Homologous Dose of NVX-CoV2373
- PMID: 36734884
- PMCID: PMC9891358
- DOI: 10.1056/NEJMc2215509
Immunogenicity of a Fourth Homologous Dose of NVX-CoV2373
Figures
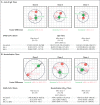
Dataset described in
-
Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2022 Nov;22(11):1565-1576. doi: 10.1016/S1473-3099(22)00420-0. Epub 2022 Aug 10. Lancet Infect Dis. 2022. PMID: 35963274 Free PMC article. Clinical Trial.
References
-
- van der Straten K, Guerra D, van Gils MJ, et al. Mapping the antigenic diversification of SARS-CoV-2. January 3, 2022. (https://www.medrxiv.org/content/10.1101/2022.01.03.21268582v1). preprint.
-
- Glenn GM. Vaccines and related biological products advisory committee. Silver Spring, MD: Food and Drug Administration, June 28, 2022. (https://www.fda.gov/media/159498/download).
-
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018;378:2346-2348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
