Pre-Equilibrium Reaction Mechanism as a Strategy to Enhance Rate and Lower Overpotential in Electrocatalysis
- PMID: 36734988
- PMCID: PMC9936576
- DOI: 10.1021/jacs.2c10942
Pre-Equilibrium Reaction Mechanism as a Strategy to Enhance Rate and Lower Overpotential in Electrocatalysis
Abstract
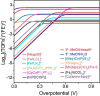
Pre-equilibrium reaction kinetics enable the overall rate of a catalytic reaction to be orders of magnitude faster than the rate-determining step. Herein, we demonstrate how pre-equilibrium kinetics can be applied to breaking the linear free-energy relationship (LFER) for electrocatalysis, leading to rate enhancement 5 orders of magnitude and lowering of overpotential to approximately thermoneutral. This approach is applied to pre-equilibrium formation of a metal-hydride intermediate to achieve fast formate formation rates from CO2 reduction without loss of selectivity (i.e., H2 evolution). Fast pre-equilibrium metal-hydride formation, at 108 M-1 s-1, boosts the CO2 electroreduction to formate rate up to 296 s-1. Compared with molecular catalysts that have similar overpotential, this rate is enhanced by 5 orders of magnitude. As an alternative comparison, overpotential is lowered by ∼50 mV compared to catalysts with a similar rate. The principles elucidated here to obtain pre-equilibrium reaction kinetics via catalyst design are general. Design and development that builds on these principles should be possible in both molecular homogeneous and heterogeneous electrocatalysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Nitopi S.; Bertheussen E.; Scott S. B.; Liu X.; Engstfeld A. K.; Horch S.; Seger B.; Stephens I. E. L.; Chan K.; Hahn C.; Nørskov J. K.; Jaramillo T. F.; Chorkendorff I. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. 10.1021/acs.chemrev.8b00705. - DOI - PubMed
-
- Olah G. A.; Goeppert A.; Surya Prakash G. K.. Beyond Oil and Gas: The Methanol Economy, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009; pp 233–288.
-
- Chen H.; Dong F.; Minteer S. D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat. Catal. 2020, 3, 225–244. 10.1038/s41929-019-0408-2. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

