Association of Anthracycline With Heart Failure in Patients Treated for Breast Cancer or Lymphoma, 1985-2010
- PMID: 36735254
- PMCID: PMC9898820
- DOI: 10.1001/jamanetworkopen.2022.54669
Association of Anthracycline With Heart Failure in Patients Treated for Breast Cancer or Lymphoma, 1985-2010
Erratum in
-
Error in Affiliations.JAMA Netw Open. 2023 Mar 1;6(3):e234015. doi: 10.1001/jamanetworkopen.2023.4015. JAMA Netw Open. 2023. PMID: 36867413 Free PMC article. No abstract available.
Abstract
Importance: Anthracyclines increase the risk for congestive heart failure (CHF); however, long-term cumulative incidence and risk factors for CHF after anthracycline therapy are not well defined in population-based studies.
Objective: To compare the long-term cumulative incidence of CHF in patients with breast cancer or lymphoma treated with anthracycline therapy compared with healthy controls from the same community.
Design, setting, and participants: This retrospective population-based case-control study included data from the Rochester Epidemiology Project. Participants included residents of Olmsted County, Minnesota, diagnosed with breast cancer or lymphoma from January 1985 through December 2010 matched for age, sex, and comorbidities with healthy controls, with a final ratio of 1 case to 1.5 controls. Statistical analysis was performed between July 2017 and February 2022.
Exposures: Cancer treatment and CHF risk factors.
Main outcomes and measures: The main outcome was new-onset CHF, as defined by the modified Framingham criteria. Cox proportional hazards regression was used to estimate hazard ratios (HRs) to compare the risk of CHF in participants with cancer vs controls, adjusted for age, sex, diabetes, hypertension, hyperlipidemia, coronary artery disease, obesity, and smoking history.
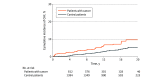
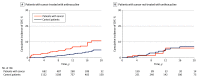
Results: A total of 2196 individuals were included, with 812 patients with cancer and 1384 participants without cancer. The mean (SD) age was 52.62 (14.56) years and 1704 participants (78%) were female. Median (IQR) follow-up was 8.6 (5.2-13.4) years in the case group vs 12.5 (8.7-17.5) years in the control group. Overall, patients with cancer had higher risk of CHF compared with the control cohort even after adjusting for age, sex, diabetes, hypertension, coronary artery disease, hyperlipidemia, obesity, and smoking status (HR, 2.86 [95% CI, 1.90-4.32]; P < .001). After adjusting for the same variables, CHF risk was greater for patients with cancer receiving anthracycline (HR, 3.25 [95% CI, 2.11-5.00]; P < .001) and was attenuated and lost statistical significance for patients with cancer not receiving anthracyclines (HR, 1.78 [95% CI, 0.83-3.81]; P = .14). Higher cumulative incidence for patients treated with anthracyclines vs comparator cohort was observed at 1 year (1.81% vs 0.09%), 5 years (2.91% vs 0.79%), 10 years (5.36% vs 1.74%), 15 years (7.42% vs 3.18%), and 20 years (10.75% vs 4.98%) (P < .001). There were no significant differences in risk of CHF for patients receiving anthracycline at a dose of less than 180 mg/m2 compared with those at a dose of 180 to 250 mg/m2 (HR, 0.54 [95% CI, 0.19-1.51]) or at a dose of more than 250 mg/m2 (HR, 1.23 [95% CI, 0.52-2.91]). At diagnosis, age was an independent risk factor associated with CHF (HR per 10 years, 2.77 [95% CI, 1.99-3.86]; P < .001).
Conclusions and relevance: In this retrospective population-based case-control study, anthracyclines were associated with an increased risk of CHF early during follow-up, and the increased risk persisted over time. The cumulative incidence of CHF in patients with breast cancer or lymphoma treated with anthracyclines at 15 years was more than 2-fold that of the control group.
Conflict of interest statement
Figures
Comment in
-
Heart Failure in Patients With Cancer Treated With Anthracyclines-Revisiting the Foundation of Cardio-Oncology.JAMA Netw Open. 2023 Feb 1;6(2):e2254677. doi: 10.1001/jamanetworkopen.2022.54677. JAMA Netw Open. 2023. PMID: 36735260 No abstract available.
References
-
- Lancellotti P, Nkomo VT, Badano LP, et al. ; European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography . Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(9):1013-1032. doi: 10.1016/j.echo.2013.07.005 - DOI - PubMed
-
- Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Cancer Epidemiology and Prevention. 4th ed. Oxford University Press; 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

