Epicardial vs. transvenous implantable cardioverter defibrillators in children
- PMID: 36735263
- PMCID: PMC10062323
- DOI: 10.1093/europace/euad015
Epicardial vs. transvenous implantable cardioverter defibrillators in children
Abstract
Aims: The implantable cardioverter defibrillator (ICD) has been increasingly used in children. Both epicardial and transvenous approaches are used, with controversy regarding the best option with no specific recommendations. We aimed to compare outcomes associated with epicardial vs. transvenous ICDs in children.
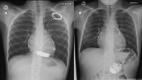
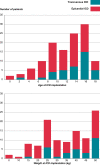
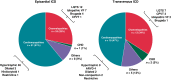
Methods and results: Data were analysed from a retrospective study including all patients <18-year-old implanted with an ICD in a tertiary centre from 2003 to 2021. Outcomes were compared between epicardial and transvenous ICDs. A total of 122 children with an ICD (mean age 11.5 ± 3.8 years, 57.4% males) were enrolled, with 84 (64.1%) epicardial ICDs and 38 (29.0%) transvenous ICDs. Early (<30 days) ICD-related complications were reported in 17 (20.2%) patients with an epicardial ICD vs. 0 (0.0%) with a transvenous ICD (P = 0.002). Over a mean follow-up of 4.8 ± 4.0 years, 25 (29.8%) patients with an epicardial ICD and 9 (23.7%) patients with a transvenous ICD experienced at least one late ICD-related complication [hazard ratio (HR) 1.8, 95% confidence interval (CI) 0.8-4.0]. Implantable cardioverter defibrillator lead dysfunction occurred in 19 (22.6%) patients with an epicardial ICD vs. 3 (7.9%) with a transvenous ICD (HR 5.7, 95% CI 1.3-24.5) and was associated with a higher incidence of ICD-related reintervention (HR 3.0, 95% CI 1.3-7.0). After considering potential confounders, especially age and weight at implantation, this association was no longer significant (P = 0.112). The freedom from ICD lead dysfunction was greater in patients with pleural coils than in those with epicardial coils (HR 0.38, 95% CI 0.15-0.96).
Conclusion: In children, after a consideration of patient characteristics at implantation, the burden of complications and ICD lead dysfunction appears to be similar in patients with epicardial and transvenous devices. Pleural coils seem to be associated with better outcomes than epicardial coils in this population.
Clinical trial registration: NCT05349162.
Keywords: Congenital heart disease; Implantable cardioverter defibrillator; Paediatric; Sudden death; Ventricular arrhythmia.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: Victor Waldmann is consultant for Abbott, Medtronic, and Withings.
Figures


References
-
- Jordan CP, Freedenberg V, Wang Y, Curtis JP, Gleva MJ, Berul CI. Implant and clinical characteristics for pediatric and congenital heart patients in the national cardiovascular data registry implantable cardioverter defibrillator registry. Circ Arrhythm Electrophysiol 2014;7:1092–100. - PubMed
-
- Baskar S, Bao H, Minges KE, Spar DS, Czosek RJ. Characteristics and outcomes of pediatric patients who undergo placement of implantable cardioverter defibrillators: insights from the National Cardiovascular Data Registry. Circ Arrhythm Electrophysiol 2018;11:e006542. - PubMed
-
- Bogush N, Espinosa RE, Cannon BC, Wackel PL, Okamura H, Friedman PAet al. Selecting the right defibrillator in the younger patient: transvenous, epicardial or subcutaneous? Int J Cardiol 2018;250:133–8. - PubMed
-
- Shah MJ, Silka MJ, Silva JNA, Balaji S, Beach CM, Benjamin MNet al. 2021 PACES expert consensus statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Heart Rhythm 2021;18:1888–924. - PubMed
-
- Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen Jet al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) working group on grown-up congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719–1753. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

