A Microsimulation Study of the Cost-Effectiveness of Hepatitis C Virus Screening Frequencies in Hemodialysis Centers
- PMID: 36735375
- PMCID: PMC10103100
- DOI: 10.1681/ASN.2022030245
A Microsimulation Study of the Cost-Effectiveness of Hepatitis C Virus Screening Frequencies in Hemodialysis Centers
Abstract
Background: National guidelines recommend twice-yearly hepatitis C virus (HCV) screening for patients receiving in-center hemodialysis. However, studies examining the cost-effectiveness of HCV screening methods or frequencies are lacking.
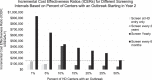
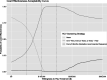
Methods: We populated an HCV screening, treatment, and disease microsimulation model with a cohort representative of the US in-center hemodialysis population. Clinical outcomes, costs, and cost-effectiveness of the Kidney Disease Improving Global Outcomes (KDIGO) 2018 guidelines-endorsed HCV screening frequency (every 6 months) were compared with less frequent periodic screening (yearly, every 2 years), screening only at hemodialysis initiation, and no screening. We estimated expected quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICERs) between each screening strategy and the next less expensive alternative strategy, from a health care sector perspective, in 2019 US dollars. For each strategy, we modeled an HCV outbreak occurring in 1% of centers. In sensitivity analyses, we varied mortality, linkage to HCV cure, screening method (ribonucleic acid versus antibody testing), test sensitivity, HCV infection rates, and outbreak frequencies.
Results: Screening only at hemodialysis initiation yielded HCV cure rates of 79%, with an ICER of $82,739 per QALY saved compared with no testing. Compared with screening at hemodialysis entry only, screening every 2 years increased cure rates to 88% and decreased liver-related deaths by 52%, with an ICER of $140,193. Screening every 6 months had an ICER of $934,757; in sensitivity analyses using a willingness-to-pay threshold of $150,000 per QALY gained, screening every 6 months was never cost-effective.
Conclusions: The KDIGO-recommended HCV screening interval (every 6 months) does not seem to be a cost-effective use of health care resources, suggesting that re-evaluation of less-frequent screening strategies should be considered.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
P.P. Reese reports consultancy: VALHealth-identification of patients with CKD and behavior change strategies; ownership interest: various equities, but none specifically health-focused and none directly related to author's research; research funding: co-principal investigator for investigator-initiated and collaborative trials with funding and/or antiviral medication supplied by AbbVie, Gilead, and Merck paid to the University of Pennsylvania to support research on transplantation of HCV-infected organs into uninfected recipients, followed by antiviral treatment; honoraria: salary from the National Kidney Foundation for work as Associate Editor of the
Figures

Comment in
-
How Frequently Should We Screen for Hepatitis C in US Hemodialysis Centers? Evaluating the Cost-Effectiveness of Different Strategies.J Am Soc Nephrol. 2023 Feb 1;34(2):193-194. doi: 10.1681/ASN.0000000000000031. J Am Soc Nephrol. 2023. PMID: 36735372 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

