Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction
- PMID: 36735787
- PMCID: PMC9897666
- DOI: 10.1126/sciadv.adc9465
Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction
Erratum in
-
Erratum for the Research Article: "Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction".Sci Adv. 2023 Dec 22;9(51):eadn2108. doi: 10.1126/sciadv.adn2108. Epub 2023 Dec 22. Sci Adv. 2023. PMID: 38134287 Free PMC article. No abstract available.
Abstract
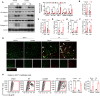
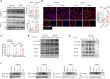
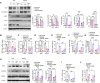
High levels of lactate are positively associated with the prognosis and mortality in patients with heart attack. Endothelial-to-mesenchymal transition (EndoMT) plays an important role in cardiac fibrosis. Here, we report that lactate exerts a previously unknown function that increases cardiac fibrosis and exacerbates cardiac dysfunction by promoting EndoMT following myocardial infarction (MI). Treatment of endothelial cells with lactate disrupts endothelial cell function and induces mesenchymal-like function following hypoxia by activating the TGF-β/Smad2 pathway. Mechanistically, lactate induces an association between CBP/p300 and Snail1, leading to lactylation of Snail1, a TGF-β transcription factor, through lactate transporter monocarboxylate transporter (MCT)-dependent signaling. Inhibiting Snail1 diminishes lactate-induced EndoMT and TGF-β/Smad2 activation after hypoxia/MI. The MCT inhibitor CHC mitigates lactate-induced EndoMT and Snail1 lactylation. Silence of MCT1 compromises lactate-promoted cardiac dysfunction and EndoMT after MI. We conclude that lactate acts as an important molecule that up-regulates cardiac EndoMT after MI via induction of Snail1 lactylation.
Figures







References
-
- S. S. Virani, A. Alonso, H. J. Aparicio, E. J. Benjamin, M. S. Bittencourt, C. W. Callaway, A. P. Carson, A. M. Chamberlain, S. Cheng, F. N. Delling, M. S. V. Elkind, K. R. Evenson, J. F. Ferguson, D. K. Gupta, S. S. Khan, B. M. Kissela, K. L. Knutson, C. D. Lee, T. T. Lewis, J. Liu, M. S. Loop, P. L. Lutsey, J. Ma, J. Mackey, S. S. Martin, D. B. Matchar, M. E. Mussolino, S. D. Navaneethan, A. M. Perak, G. A. Roth, Z. Samad, G. M. Satou, E. B. Schroeder, S. H. Shah, C. M. Shay, A. Stokes, L. VanWagner, N. Y. Wang, C. W. Tsao; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 143, e254–e743 (2021). - PubMed
-
- S. Sahoo, D. W. Losordo, Exosomes and cardiac repair after myocardial infarction. Circ. Res. 114, 333–344 (2014). - PubMed
-
- A. Segura, O. Frazier, L. Buja, Fibrosis and heart failure. Heart Fail. Rev. 19, 173–185 (2014). - PubMed
-
- A. Leask, Getting to the heart of the matter: New insights into cardiac fibrosis. Circ. Res. 116, 1269–1276 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

