Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study
- PMID: 36735893
- PMCID: PMC10082300
- DOI: 10.1200/JCO.22.01990
Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study
Abstract
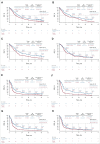
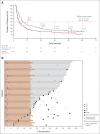
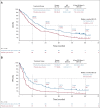
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.We report 5-year efficacy and safety outcomes from the phase III KEYNOTE-407 study (ClinicalTrials.gov identifier: NCT02775435). Eligible patients with previously untreated, metastatic squamous non-small-cell lung cancer (NSCLC) were randomly assigned 1:1 to pembrolizumab 200 mg or placebo plus carboplatin and paclitaxel/nab-paclitaxel once every 3 weeks for four cycles, followed by pembrolizumab or placebo for up to 35 cycles. Primary end points were overall survival (OS) and progression-free survival (PFS) per RECIST version 1.1 by blinded independent central review (BICR). Five hundred fifty-nine patients were randomly assigned in the intention-to-treat population (pembrolizumab plus chemotherapy, n = 278; placebo plus chemotherapy, n = 281). The median time from random assignment to data cutoff was 56.9 (range, 49.9-66.2) months. OS and PFS were improved with pembrolizumab plus chemotherapy versus placebo plus chemotherapy (hazard ratio [95% CI], 0.71 [0.59 to 0.85] and 0.62 [0.52 to 0.74]), with 5-year OS rates of 18.4% versus 9.7%, respectively. Toxicity was manageable. Among 55 patients who completed 35 cycles of pembrolizumab, the objective response rate was 90.9% and the 3-year OS rate after completion of 35 cycles (approximately 5 years after random assignment) was 69.5%. Pembrolizumab plus chemotherapy maintained an OS and PFS benefit versus placebo plus chemotherapy in previously untreated, metastatic squamous NSCLC and is a standard-of-care first-line treatment option for metastatic squamous NSCLC regardless of programmed death ligand 1 expression.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
Comment in
-
KEYNOTE-407: an effective and safe first-line treatment option for metastatic squamous non-small cell lung cancer.Transl Lung Cancer Res. 2023 Aug 30;12(8):1830-1833. doi: 10.21037/tlcr-23-271. Epub 2023 Jul 19. Transl Lung Cancer Res. 2023. PMID: 37691862 Free PMC article. No abstract available.
-
Building on success: key takeaways from the 5-year update of the KEYNOTE-407 study.Transl Lung Cancer Res. 2023 Aug 30;12(8):1812-1815. doi: 10.21037/tlcr-23-290. Epub 2023 Aug 15. Transl Lung Cancer Res. 2023. PMID: 37691870 Free PMC article. No abstract available.
References
-
- Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 - PubMed
-
- Paz-Ares L, Vicente D, Tafreshi A, et al. : A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657-1669, 2020 - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. : Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92-98, 2002 - PubMed

