Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated With a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter inspIRE Study
- PMID: 36735937
- PMCID: PMC10026968
- DOI: 10.1161/CIRCEP.122.011780
Paroxysmal Atrial Fibrillation Ablation Using a Novel Variable-Loop Biphasic Pulsed Field Ablation Catheter Integrated With a 3-Dimensional Mapping System: 1-Year Outcomes of the Multicenter inspIRE Study
Abstract
Background: The inspIRE study (Study for Treatment of Paroxysmal Atrial Fibrillation [PAF] by Pulsed Field Ablation [PFA] System With Irreversible Electroporation [IRE]) evaluated safety and effectiveness of a fully integrated biphasic pulsed field ablation (PFA) system with a variable-loop circular catheter for the treatment of drug-refractory paroxysmal atrial fibrillation.
Methods: Subjects underwent pulmonary vein (PV) isolation with the PFA system, using at least 12 applications per vein; adenosine/isoproterenol was administered to confirm entrance block. Wave I assessed initial safety, including for esophageal lesions, silent cerebral lesions, and PV stenosis. Wave II (pivotal phase) tested (1) primary safety, incidence of early-onset primary adverse events, and (2) primary effectiveness, confirmed PV isolation with freedom from documented atrial arrhythmia at 12 months. The study design specified an interim analysis to determine early success once 30 subjects reached the 12-month follow-up and all subjects reached 3-month follow-up.
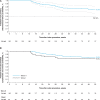
Results: Across 13 centers in Europe/Canada, 226 subjects were enrolled, met criteria for safety and effectiveness evaluations, and received PFA (Wave I, 40; Wave II, 186). Wave I demonstrated no esophageal thermal lesions or PV stenosis. Among 39 subjects with cerebral magnetic resonance imaging, silent cerebral lesions were detected in 4 of the first 6 subjects, after which workflow enhancements, including a 10-second pause between PFA applications, were implemented; subsequently, only 4 of 33 subjects had silent cerebral lesions. In the Wave II phase, no primary adverse events were reported. Upon declaring early success, 83 subjects reached 12-month follow-up. With 100% entrance block, PV isolation without acute reconnection was achieved in 97.1% of targeted veins. For Wave II, the primary effectiveness end point per Kaplan-Meier at the time of interim analysis was 70.9%; 12-month freedom from symptomatic atrial fibrillation/atrial flutter/atrial tachycardia recurrence and repeat ablation was 78.9% and 92.3%, respectively. Total procedure and transpired PFA times were 70.1±27.7 and 26.7±14.0 minutes, respectively.
Conclusions: The inspIRE trial confirmed the safety and effectiveness of the novel mapping-integrated PFA system.
Registration: URL: https://www.
Clinicaltrials: gov; unique identifier: NCT04524364.
Keywords: atrial fibrillation; catheter ablation; efficacy; electroporation; radiofrequency ablation.
Conflict of interest statement
Dr Duytschaever has served on the speaker’s bureau, is a consultant for Biosense Webster, Inc, and has received research support from Biosense Webster, Inc. Dr De Potter has received consulting fees, honorarium for lectures, and presentation fees from Biosense Webster and Adagio Medical (all payments were directed to the institution). Dr Anic has received consulting fees and has contracted research with Farapulse, Boston Scientific, Galaxy Medical, and Biosense Webster. Dr Grimaldi has an unrelated patent agreement with Biosense Webster, Inc. Dr Neuzil has received grant support from Biosense Webster, Inc. Dr Van Herendael has received support from Biosense Webster for congress-related activities. Dr Verma has received grants from Biosense Webster, Medtronic, Bayer, and Biotronik; has received consulting fees from Biosense Webster, Medtronic, Adagio Medical, Galaxy Medical, Ablacon, and Thermedical; and has received honorarium for lectures from Biosense Webster and Medtronic. Dr Skanes has served on the speaker’s bureau for Biosense Webster and has received research support from Biosense Webster, Inc. Dr Scherr has received grant support from Biosense Webster. Dr Pürerfellner has received consulting fees from Biosense Webster, Abbott, Boston Scientific, Biotronik, and Medtronic and has received payment or honorarium for lectures or presentations from Biosense Webster, Abbott, Boston Scientific, Biotronik, and Medtronic. Dr Jais has received a LIRYC research grant from Biosense Webster; has received speaker fees from Biosense Webster; is a share holder of Farapulse/Affera; and has also received speaker fees and research grants from Boston Scientific, Medtronic, and Abbott. Dr Reddy is a consultant for Biosense Webster, Inc; he also has additional disclosures unrelated to this article that are listed in the
Figures

References
-
- Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005 - PubMed
-
- Verma A, Boersma L, Haines DE, Natale A, Marchlinski FE, Sanders P, Calkins H, Packer DL, Hummel J, Onal B, et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrhythm Electrophysiol. 2022;15:e010168. doi: 10.1161/CIRCEP.121.010168 - PMC - PubMed
-
- Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol. 2019;12:e007781. doi: 10.1161/CIRCEP.119.007781 - PMC - PubMed
-
- Stewart MT, Haines DE, Verma A, Kirchhof N, Barka N, Grassl E, Howard B. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–764. doi: 10.1016/j.hrthm.2018.10.030 - PubMed
-
- Yavin H, Brem E, Zilberman I, Shapira-Daniels A, Datta K, Govari A, Anic A, Wazni O, Anter E. Circular multielectrode pulsed field ablation catheter Lasso pulsed field ablation: lesion characteristics, durability and effect on neighboring structures. Circ Arrhythm Electrophysiol. 2021;14:e009229. doi: 10.1161/CIRCEP.120.009229 - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

