Alcohol Dependence Modifies Brain Networks Activated During Withdrawal and Reaccess: A c-Fos-Based Analysis in Mice
- PMID: 36736419
- PMCID: PMC10517410
- DOI: 10.1016/j.biopsych.2023.01.018
Alcohol Dependence Modifies Brain Networks Activated During Withdrawal and Reaccess: A c-Fos-Based Analysis in Mice
Abstract
Background: High-level alcohol consumption causes neuroplastic changes in the brain that promote pathological drinking behavior. Some of these changes have been characterized in defined brain circuits and cell types, but unbiased approaches are needed to explore broader patterns of adaptations.
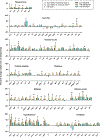
Methods: We used whole-brain c-Fos mapping and network analysis to assess patterns of neuronal activity during alcohol withdrawal and following reaccess in a well-characterized model of alcohol dependence. Mice underwent 4 cycles of chronic intermittent ethanol to increase voluntary alcohol consumption, and a subset underwent forced swim stress to further escalate consumption. Brains were collected either 24 hours (withdrawal) or immediately following a 1-hour period of alcohol reaccess. c-fos counts were obtained for 110 brain regions using iDISCO and ClearMap. Then, we classified mice as high or low drinkers and used graph theory to identify changes in network properties associated with high-drinking behavior.
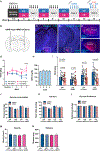
Results: During withdrawal, chronic intermittent ethanol mice displayed widespread increased c-Fos expression relative to air-exposed mice, independent of forced swim stress. Reaccess drinking reversed this increase. Network modularity, a measure of segregation into communities, was increased in high-drinking mice after alcohol reaccess relative to withdrawal. The cortical amygdala showed increased cross-community coactivation during withdrawal in high-drinking mice, and cortical amygdala silencing in chronic intermittent ethanol mice reduced voluntary drinking.
Conclusions: Alcohol withdrawal in dependent mice causes changes in brain network organization that are attenuated by reaccess drinking. Olfactory brain regions, including the cortical amygdala, drive some of these changes and may play an important but underappreciated role in alcohol dependence.
Keywords: Alcohol; Chronic intermittent ethanol; Graph theory; Mice; Networks; c-Fos.
Copyright © 2023 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Figures


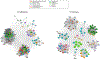


Comment in
-
Unveiling New Brain Circuits and Network Activity in Alcohol Use Disorder: Insights From Innovative Technologies.Biol Psychiatry. 2023 Sep 1;94(5):365-366. doi: 10.1016/j.biopsych.2023.06.020. Biol Psychiatry. 2023. PMID: 37558313 No abstract available.
References
-
- Becker HC (2013): Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci 13: 355–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

