Exploring monitoring strategies for population surveillance of HPV vaccine impact using primary HPV screening
- PMID: 36736490
- PMCID: PMC9925607
- DOI: 10.1016/j.tvr.2023.200255
Exploring monitoring strategies for population surveillance of HPV vaccine impact using primary HPV screening
Abstract
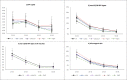
Australia's cervical screening program transitioned from cytology to HPV-testing with genotyping for HPV16/18 in Dec'2017. We investigated whether program data could be used to monitor HPV vaccination program impact (commenced in 2007) on HPV16/18 prevalence and compared estimates with pre-vaccination benchmark prevalence. Pre-vaccination samples (2005-2008) (n = 1933; WHINURS), from 25 to 64-year-old women had been previously analysed with Linear Array (LA). Post-vaccination samples (2013-2014) (n = 2989; Compass pilot), from 25 to 64-year-old women, were analysed by cobas 4800 (cobas), and by LA for historical comparability. Age standardised pre-vaccination HPV16/18 prevalence was 4.85% (95%CI:3.81-5.89) by LA; post-vaccination estimates were 1.67% (95%CI:1.21-2.13%) by LA, 1.49% (95%CI:1.05-1.93%) by cobas, and 1.63% (95%CI:1.17-2.08%) for cobas and LA testing of non-16/18 cobas positives (cobas/LA). Age-standardised pre-vaccination oncogenic HPV prevalence was 15.70% (95%CI:13.79-17.60%) by LA; post-vaccination estimates were 9.06% (95%CI:8.02-10.09%) by LA, 8.47% (95%CI:7.47-9.47%) by cobas and cobas/LA. Standardised rate ratios between post-vs. pre-vaccination rates were significantly different for HPV16/18, non-16/18 HPV and oncogenic HPV: 0.34 (95%CI:0.23-0.50), 0.68 (95%CI:0.55-0.84) and 0.58 (95%CI:0.48-0.69), respectively. Additional strategies (LA for all cobas positives; combined cobas and LA results on all samples) had similar results. If a single method is applied consistently, it will provide important data on relative changes in HPV prevalence following vaccination.
Keywords: Cobas 4800; HPV vaccination; HPV-Based screening; Linear array; Prevalence; Surveillance.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: KC and MS are co-PIs, and MC is an investigator, of an investigator-initiated trial of cervical screening, Compass, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), a government-funded not-for-profit charity. Compass receives infrastructure support from the Australian government and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. KC is also co-PI on a major implementation program Elimination of Cervical Cancer in the Western Pacific which has received support from the Minderoo Foundation and the Frazer Family Foundation and equipment donations from Cepheid Inc. KC receives contract funding from Commonwealth Department of Health, Australia to her institution for work to monitor the safety of the National Cervical Screening Program. KC also receives support for a range of other Australian and international government projects including support from philanthropic organizations, WHO, and government agencies related to cervical cancer control.
MS, DH, JMLB, and KAK state receiving free testing kit donations for research purposes from some of the following comparies: Roche, Seegene, Abbott, Becton Dickinson, Cepheid, AusDiganostics, Copan, Qiagen and Atila Biosystems. MAS receives salary support via fellowship grants from the National Health and Medical Research Council (NHMRC) of Australia and Cancer Institute NSW and contracts paid to her institution (the Daffodil Centre) with the Commonwealth Department of Health (Australia) and National Screening Unit (New Zealand). MS holds NHMRC grants for 5 projects, is the Director for Cancer Council Australia and Co-chair of HPV test characteristics expert panel and consultant for Cancer Care Ontario. DH reports payment to his institution by Roche for presentations, lectures, etc; conference registration fees paid by Roche, Abbott and Qiagen and membership of the Quality and Safety monitoring committee for the Australian National Cervical Screening Program (unpaid). PEC reports receipt of HPV tests and assays at a reduced or no cost for research only from Roche, Atila, Cepheid, Becton Dickinson, and Arbor Vita. SMG reports an an NHMRC Leadership Investigator grant, an investigator-initiated grant to her institution on HPV in young women; lecture fees from Merck for work performed in personal time; participation in the Global Advisory Board on HPV by Merck; being past and inaugural president of the Asia Oceania research 396 organization on Genital Infections and Neoplasia (AOGIN) and Vice President of the International Papillomavirus Society (IPVS) (unpaid). CMW reports grants to her institutions from the US National Institute of Allergy and Infectious Diseases, the US National Cancer Institute, Hologic and Becton Dickinson; receiving reagents and equipment from Roche Molecular Systems and Roche/Ventana Medical Systems through her institution outside the submitted work and personal fees from Becton Dickinson outside the submitted work. CDW is Deputy Chair of The Australian Centre for the Prevention of Cervical Cancer (formerly VCS Foundation Pty Ltd); owns shares in CSL Pty Ltd; has received Honoraria from BioGen, Merck and Seqirus and sponsorship to attend EOGIN 2019 from Seqirus; is a member of the Quality and Safety Monitoring Committee of the National Cervical Screening Program. LSV and JT have no conflicts of interests to declare.
Figures
References
-
- Australian Government, Department of Health Historical human papillomavirus (HPV) immunisation coverage rates. Historical reports showing vaccination coverage with 3 doses of the HPV vaccine for adolescents turning 15 years of age by year for each state and territory. https://www.health.gov.au/sites/default/files/documents/2019/12/historic...
-
- Australian Government, Department of Health National HPV vaccination coverage for the female catch up cohort by year of age. Historical reports showing national human papillomavirus (HPV) vaccination coverage for the female catch up cohort by dose number (1, 2 or 3) and single age cohort (12–26 years) for the specified year. https://www.health.gov.au/sites/default/files/documents/2019/12/national...
-
- Smith M.A., Liu B., McIntyre P., Menzies R., Dey A., Canfell K. Fall in genital warts diagnoses in the general and indigenous Australian population following implementation of a national human papillomavirus vaccination program: analysis of routinely collected national hospital data. J. Infect. Dis. 2015;211:91–99. - PubMed
-
- Australian Institute of Health and Welfare National cervical screening program monitoring report 2019. 2019. https://www.aihw.gov.au/getmedia/fcacac12-cd05-4325-88bc-5529a61b53f3/ai... Cat. no. CAN 132. Canberra: AIHW.