IL-13-associated epithelial remodeling correlates with clinical severity in nasal polyposis
- PMID: 36736797
- PMCID: PMC10243183
- DOI: 10.1016/j.jaci.2022.12.826
IL-13-associated epithelial remodeling correlates with clinical severity in nasal polyposis
Abstract
Background: Epithelial remodeling is a histopathologic feature of chronic inflammatory airway diseases including chronic rhinosinusitis (CRS). Cell-type shifts and their relationship to CRS endotypes and severity are incompletely described.
Objective: We sought to understand the relationship of epithelial cell remodeling to inflammatory endotypes and disease outcomes in CRS.
Methods: Using cell-type transcriptional signatures derived from epithelial single-cell sequencing, we analyzed bulk RNA-sequencing data from sinus epithelial brushings obtained from patients with CRS with and without nasal polyps in comparison to healthy controls.
Results: The airway epithelium in nasal polyposis displayed increased tuft cell transcripts and decreased ciliated cell transcripts along with an IL-13 activation signature. In contrast, CRS without polyps showed an IL-17 activation signature. IL-13 activation scores were associated with increased tuft cell, goblet cell, and mast cell scores and decreased ciliated cell scores. Furthermore, the IL-13 score was strongly associated with a previously reported activated ("polyp") tuft cell score and a prostaglandin E2 activation signature. The Lund-Mackay score, a computed tomographic metric of sinus opacification, correlated positively with activated tuft cell, mast cell, prostaglandin E2, and IL-13 signatures and negatively with ciliated cell transcriptional signatures.
Conclusions: These results demonstrate that cell-type alterations and prostaglandin E2 stimulation are key components of IL-13-induced epithelial remodeling in nasal polyposis, whereas IL-17 signaling is more prominent in CRS without polyps, and that clinical severity correlates with the degree of IL-13-driven epithelial remodeling.
Keywords: Chronic rhinosinusitis; IL-13; endotype; epithelial remodeling; nasal polyposis; prostaglandin E2; type 2 inflammation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure of potential conflict of interest: J. G. Gurrola II is a Genentech Advisory Board Consultant with agreement ending December 2021. A. N. Goldberg is a minor stock holder in Siesta Medical. S. D. Pletcher and A. N. Goldberg are coinventors of patent 14/394, 006 Sinus diagnostics and treatments. The rest of the authors declare that they have no relevant conflicts of interests.
Figures
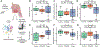

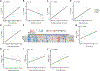

References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL107202/HL/NHLBI NIH HHS/United States
- R01 AI136962/AI/NIAID NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- R01 HL135156/HL/NHLBI NIH HHS/United States
- U19 AI077439/AI/NIAID NIH HHS/United States
- K08 HL155490/HL/NHLBI NIH HHS/United States
- F32 HL158174/HL/NHLBI NIH HHS/United States
- F32 HL140868/HL/NHLBI NIH HHS/United States
- R01 HL128439/HL/NHLBI NIH HHS/United States
- P01 HL132821/HL/NHLBI NIH HHS/United States
- R15 AI147148/AI/NIAID NIH HHS/United States
- T32 HL007185/HL/NHLBI NIH HHS/United States

