Topical captopril: a promising treatment for secondary lymphedema
- PMID: 36736951
- PMCID: PMC10192126
- DOI: 10.1016/j.trsl.2023.01.005
Topical captopril: a promising treatment for secondary lymphedema
Abstract
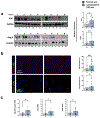
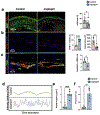
Transforming growth factor-beta 1 (TGF-β1)-mediated tissue fibrosis is an important regulator of lymphatic dysfunction in secondary lymphedema. However, TGF-β1 targeting can cause toxicity and autoimmune complications, limiting clinical utility. Angiotensin II (Ang II) modulates intracellular TGF-β1 signaling, and inhibition of Ang II production using angiotensin-converting enzyme (ACE) inhibitors, such as captopril, has antifibrotic efficacy in some pathological settings. Therefore, we analyzed the expression of ACE and Ang II in clinical lymphedema biopsy specimens from patients with unilateral breast cancer-related lymphedema (BCRL) and mouse models, and found that cutaneous ACE expression is increased in lymphedematous tissues. Furthermore, topical captopril decreases fibrosis, activation of intracellular TGF-β1 signaling pathways, inflammation, and swelling in mouse models of lymphedema. Captopril treatment also improves lymphatic function and immune cell trafficking by increasing collecting lymphatic pumping. Our results show that the renin-angiotensin system in the skin plays an important role in the regulation of fibrosis in lymphedema, and inhibition of this signaling pathway may hold merit for treating lymphedema.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures



References
-
- Rockson SG and Rivera KK, Estimating the population burden of lymphedema. Ann N Y Acad Sci, 2008. 1131: p. 147–54. - PubMed
-
- Cormier JN, et al., Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer, 2010. 116(22): p. 5138–49. - PubMed
-
- Dayan JH, et al., Lymphedema: Pathogenesis and Novel Therapies. Annu Rev Med, 2018. 69: p. 263–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

