Introduction of the Ellavi uterine balloon tamponade into the Kenyan and Ghanaian maternal healthcare package for improved postpartum haemorrhage management: an implementation research study
- PMID: 36737079
- PMCID: PMC9900048
- DOI: 10.1136/bmjopen-2022-066907
Introduction of the Ellavi uterine balloon tamponade into the Kenyan and Ghanaian maternal healthcare package for improved postpartum haemorrhage management: an implementation research study
Abstract
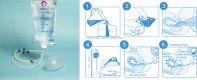
Objectives: Use of intrauterine balloon tamponades for refractory postpartum haemorrhage (PPH) management has triggered recent debate since effectiveness studies have yielded conflicting results. Implementation research is needed to identify factors influencing successful integration into maternal healthcare packages. The Ellavi uterine balloon tamponade (UBT) (Ellavi) is a new low-cost, preassembled device for treating refractory PPH.
Design: A mixed-methods, prospective, implementation research study examining the adoption, sustainability, fidelity, acceptability and feasibility of introducing a newly registered UBT. Cross-sectional surveys were administered post-training and post-use over 10 months.
Setting: Three Ghanaian (district, regional) and three Kenyan (levels 4-6) healthcare facilities.
Participants: Obstetric staff (n=451) working within participating facilities.
Intervention: PPH management training courses were conducted with obstetric staff.
Primary and secondary outcome measures: Facility measures of adoption, sustainability and fidelity and individual measures of acceptability and feasibility.
Results: All participating hospitals adopted the device during the study period and the majority (52%-62%) of the employed obstetric staff were trained on the Ellavi; sustainability and fidelity to training content were moderate. The Ellavi was suited for this context due to high delivery and PPH burden. Dynamic training curriculums led by local UBT champions and clear instructions on the packaging yielded positive attitudes and perceptions, and high user confidence, resulting in overall high acceptability. Post-training and post-use, ≥79% of the trainees reported that the Ellavi was easy to use. Potential barriers to use included the lack of adjustable drip stands and difficulties calculating bag height according to blood pressure. Overall, the Ellavi can be feasibly integrated into PPH care and was preferred over condom catheters.
Conclusions: The training package and time saving Ellavi design facilitated its adoption, acceptability and feasibility. The Ellavi is appropriate and feasible for use among obstetric staff and can be successfully integrated into the Kenyan and Ghanaian maternal healthcare package.
Trial registration numbers: NCT04502173; NCT05340777.
Keywords: ACCIDENT & EMERGENCY MEDICINE; International health services; MEDICAL EDUCATION & TRAINING; OBSTETRICS; Reproductive medicine.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Over the last decade, MP, JS, PC, PD, RO, AS, MM and EA-H have been directly involved in the development, introduction and scale of the Ellavi uterine balloon tamponade as part of their employment at PATH, which constitutes a non-financial conflict of interest. PATH, an international non-governmental organisation, did not receive any funding from Sinapi Biomedical to conduct this multisite study. The remaining authors have no conflicts of interest to declare.
Figures
References
-
- WHO . Who recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: who, 2012. Available: https://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng... [Accessed 24 June 2022]. - PubMed
-
- WHO . Who recommendations: Uterotonics for the prevention of postpartum haemorrhage. Geneva: who, 2018. Available: https://apps.who.int/iris/bitstream/handle/10665/277276/9789241550420-en... [Accessed 24 June 2022]. - PubMed
-
- Ghana Ministry of Health (MOH) . Ghana Adapted FIGO Post-Partum Haemorrhage Protocol and Care Pathways. Ministry of Health, Republic of Ghana, 2021.
-
- Kenya MOH. National annual maternal and perinatal death surveillance and response report. first report. Nairobi, Kenya: Kenya National MPDSR Secretariat, Division of Reproductive and Maternal Health, Department of Family Health, Ministry of Health Afya House, 2020.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical