The Effect of Heparin Full-Dose Anticoagulation on Survival of Hospitalized, Non-critically Ill COVID-19 Patients: A Meta-analysis of High Quality Studies
- PMID: 36738324
- PMCID: PMC9899107
- DOI: 10.1007/s00408-023-00599-6
The Effect of Heparin Full-Dose Anticoagulation on Survival of Hospitalized, Non-critically Ill COVID-19 Patients: A Meta-analysis of High Quality Studies
Abstract
Background: International COVID-19 guidelines recommend thromboprophylaxis for non-critically ill inpatients to prevent thrombotic complications. It is still debated whether full-dose thromboprophylaxis reduces all-cause mortality. The main aim of this updated systematic review and meta-analysis is to evaluate the effect of full-dose heparin-based thromboprophylaxis on survival in hospitalized non-critically ill COVID-19 patients.
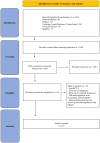
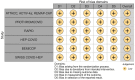
Methods: A systematic review was performed across Pubmed/Medline, EMBASE, Cochrane Central Register of clinical trials, Clinicaltrials.gov, and medRxiv.org from inception to November 2022. We conducted a meta-analysis of randomized clinical trials (RCTs) comparing full-dose heparin-based anticoagulation to prophylactic or intermediate dose anticoagulation or standard treatment in hospitalized non-critically ill COVID-19 patients. The risk of bias was assessed using the Cochrane risk-of-bias tool for randomized trials and Grading of Recommendations Assessment, Development and Evaluation was applied. The primary outcome was all-cause mortality at the longest follow-up available.
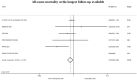
Results: We identified 6 multicenter RCTs involving 3297 patients from 13 countries across 4 continents. The rate of all-cause mortality was 6.2% (103/1662) in the full-dose group vs 7.7% (126/1635) in the prophylactic or intermediate dose group (Risk Ratio [RR] = 0.76; 95% confidence interval [CI] = 0.59-0.98; P = 0.037). The probabilities of any mortality difference and of NNT ≤ 100 were estimated at 98.2% and 84.5%, respectively. The risk of bias was low for all included RCTs and the strength of the evidence was "moderate."
Conclusion: Our meta-analysis of high-quality multicenter RCTs suggests that full-dose anticoagulation with heparin or low molecular weight heparin reduces all-cause mortality in hospitalized non-critically ill COVID-19 patients.
Study registration: PROSPERO, review no. CRD42022348993.
Keywords: Anticoagulant; COVID-19; Heparin; Hospital mortality; LMWH; SARS-CoV-2.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures


References
-
- World Health Organization (WHO) Coronavirus (COVID-19) dashboard: weekly epidemiological updates until January 9, 2023. https://covid19.who.int/. Accessed 09 Jan 2023
-
- Wieteska-Miłek M, Kuśmierczyk-Droszcz B, Ryczek R, Szmit S, Florczyk M, Mańczak R, Betkier-Lipińska K, Hoffman P, Krzesiński P, Torbicki A, Kurzyna M. Outcomes of COVID-19 in patients vaccinated and unvaccinated against SARS-CoV-2 and suffering from pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Pol Arch Intern Med. 2023;5:16406. doi: 10.20452/pamw.16406. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

