A synthetic organelle approach to probe SNARE-mediated membrane fusion in a bacterial host
- PMID: 36738791
- PMCID: PMC10011478
- DOI: 10.1016/j.jbc.2023.102974
A synthetic organelle approach to probe SNARE-mediated membrane fusion in a bacterial host
Abstract
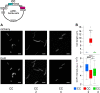
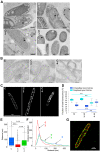
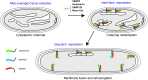
In vivo and in vitro assays, particularly reconstitution using artificial membranes, have established the role of synaptic soluble N-Ethylmaleimide-sensitive attachment protein receptors (SNAREs) VAMP2, Syntaxin-1A, and SNAP-25 in membrane fusion. However, using artificial membranes requires challenging protein purifications that could be avoided in a cell-based assay. Here, we developed a synthetic biological approach based on the generation of membrane cisternae by the integral membrane protein Caveolin in Escherichia coli and coexpression of SNAREs. Syntaxin-1A/SNAP-25/VAMP-2 complexes were formed and regulated by SNARE partner protein Munc-18a in the presence of Caveolin. Additionally, Syntaxin-1A/SNAP-25/VAMP-2 synthesis provoked increased length of E. coli only in the presence of Caveolin. We found that cell elongation required SNAP-25 and was inhibited by tetanus neurotoxin. This elongation was not a result of cell division arrest. Furthermore, electron and super-resolution microscopies showed that synaptic SNAREs and Caveolin coexpression led to the partial loss of the cisternae, suggesting their fusion with the plasma membrane. In summary, we propose that this assay reconstitutes membrane fusion in a simple organism with an easy-to-observe phenotype and is amenable to structure-function studies of SNAREs.
Keywords: SNARE proteins; caveolin; membrane fusion; membrane reconstitution; synthetic biology.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Chen A.H., Silver P.A. Designing biological compartmentalization. Trends Cell Biol. 2012;22:662–670. - PubMed
-
- Ganzinger K.A., Schwille P. More from less - bottom-up reconstitution of cell biology. J. Cell Sci. 2019;132:jcs227488. - PubMed
-
- Majumder S., Willey P.T., DeNies M.S., Liu A.P., Luxton G.W.G. A synthetic biology platform for the reconstitution and mechanistic dissection of LINC complex assembly. J. Cell Sci. 2018;132:jcs219451. - PubMed
-
- Vogel S.K., Schwille P. Minimal systems to study membrane-cytoskeleton interactions. Curr. Opin. Biotechnol. 2012;23:758–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

