Evaluation of darunavir-derived HIV-1 protease inhibitors incorporating P2' amide-derivatives: Synthesis, biological evaluation and structural studies
- PMID: 36738797
- PMCID: PMC10061991
- DOI: 10.1016/j.bmcl.2023.129168
Evaluation of darunavir-derived HIV-1 protease inhibitors incorporating P2' amide-derivatives: Synthesis, biological evaluation and structural studies
Abstract
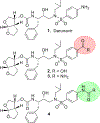
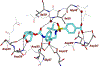
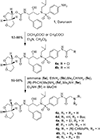
We report here the synthesis and biological evaluation of darunavir derived HIV-1 protease inhibitors and their functional effect on enzyme inhibition and antiviral activity in MT-2 cell lines. The P2' 4-amino functionality was modified to make a number of amide derivatives to interact with residues in the S2' subsite of the HIV-1 protease active site. Several compounds exhibited picomolar enzyme inhibitory and low nanomolar antiviral activity. The X-ray crystal structure of the chloroacetate derivative bound to HIV-1 protease was determined. Interestingly, the active chloroacetate group converted to the acetate functionality during X-ray exposure. The structure revealed that the P2' carboxamide functionality makes enhanced hydrogen bonding interactions with the backbone atoms in the S2'-subsite.
Keywords: Darunavir; HIV-1 protease; Inhibitor; Synthesis; X-ray structure.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
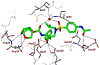

References
-
- Esté JA; Cihlar TC Current status and challenges of antiretroviral research and therapy. Antivir. Res. 2010, 85, 25–33. - PubMed
-
- Diffenbach CW; Fauci AS Thirty years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 2011, 154, 766–771. - PubMed
-
- Ghosh AK; Chapsal BD Design of the anti-HIV protease inhibitor darunavir. In ‘From Introduction to Biological and Small Molecule Drug Research and Development’ Ed. Ganellin CR; Roberts SM; Jefferis R 2013, 355–384.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

