JAK-STAT inhibition reduces endothelial prothrombotic activation and leukocyte-endothelial proadhesive interactions
- PMID: 36738826
- PMCID: PMC10246778
- DOI: 10.1016/j.jtha.2023.01.027
JAK-STAT inhibition reduces endothelial prothrombotic activation and leukocyte-endothelial proadhesive interactions
Abstract
Background: Vascular activation is characterized by increased proinflammatory, pro thrombotic, and proadhesive signaling. Several chronic and acute conditions, including Bcr-abl-negative myeloproliferative neoplasms (MPNs), graft-vs-host disease, and COVID-19 have been noted to have increased activation of the janus kinase (JAK)-signal transducer and downstream activator of transcription (STAT) pathways. Two notable inhibitors of the JAK-STAT pathway are ruxolitinib (JAK1/2 inhibitor) and fedratinib (JAK2 inhibitor), which are currently used to treat MPN patients. However, in some conditions, it has been noted that JAK inhibitors can increase the risk of thromboembolic complications.
Objectives: We sought to define the anti-inflammatory and antithrombotic effects of JAK-STAT inhibitors in vascular endothelial cells.
Methods: We assessed endothelial activation in the presence or absence of ruxolitinib or fedratinib by using immunoblots, immunofluorescence, qRT-PCR, and function coagulation assays. Finally, we used endothelialized microfluidics perfused with blood from normal and JAK2V617F+ individuals to evaluate whether ruxolitinib and fedratinib changed cell adhesion.
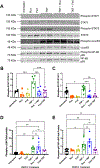
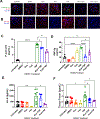
Results: We found that both ruxolitinib and fedratinib reduced endothelial cell phospho-STAT1 and STAT3 signaling and attenuated nuclear phospho-NK-κB and phospho-c-Jun localization. JAK-STAT inhibition also limited secretion of proadhesive and procoagulant P-selectin and von Willebrand factor and proinflammatory IL-6. Likewise, we found that JAK-STAT inhibition reduced endothelial tissue factor and urokinase plasminogen activator expression and activity.
Conclusions: By using endothelialized microfluidics perfused with whole blood samples, we demonstrated that endothelial treatment with JAK-STAT inhibitors prevented rolling of both healthy control and JAK2V617F MPN leukocytes. Together, these findings demonstrate that JAK-STAT inhibitors reduce the upregulation of critical prothrombotic pathways and prevent increased leukocyte-endothelial adhesion.
Keywords: endothelium; janus kinase inhibitors; myeloproliferative neoplasm; thrombosis; tissue factor.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests J.D.B. receives funds from Bayer independent from work herein. G.M.V. receives research funding from CSL Behring and Mitobridge (Astellas). C.B. has received reagents from CTI BioPharma for the conduct of clinical trial NCT 02891603 and a pending patent, WO2017058950A1, for methods of treating transplant rejection. B.C.B. holds patents related to CD4+ T cell pSTAT3 as a marker and therapeutic target of acute GVHD (WO2015120436A2); for the use of JAK inhibitors for rejection and GVHD prevention (WO2017058950A1); and for the use of CD83-targeted chimeric antigen receptor T cells in GVHD prevention, immune tolerance, autoimmunity, and acute myeloid leukemia therapy (WO2019165156). At this time, neither B.C.B. nor the University of Minnesota has received payment related to claims described in the patent. B.C.B. has received honoraria for participating in advisory board discussions for Incyte Corp and CTI BioPharma within the past 5 years. Remaining authors have no conflicts to disclose.
Figures


References
-
- Cordua S, Kjaer L, Skov V, Pallisgaard N, Hasselbalch HC, Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134:469–79. - PubMed
-
- Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595–600. - PubMed
-
- Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

