Endothelial Toll-like receptor 4 is required for microglia activation in the murine retina after systemic lipopolysaccharide exposure
- PMID: 36739425
- PMCID: PMC9899393
- DOI: 10.1186/s12974-023-02712-1
Endothelial Toll-like receptor 4 is required for microglia activation in the murine retina after systemic lipopolysaccharide exposure
Abstract
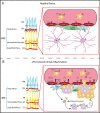
Background: Clustering of microglia around the vasculature has been reported in the retina and the brain after systemic administration of lipopolysaccharides (LPS) in mice. LPS acts via activation of Toll-like receptor 4 (TRL4), which is expressed in several cell types including microglia, monocytes and vascular endothelial cells. The purpose of this study was to investigate the effect of systemic LPS in the pigmented mouse retina and the involvement of endothelial TLR4 in LPS-induced retinal microglia activation.
Methods: C57BL/6J, conditional knockout mice that lack Tlr4 expression selectively on endothelial cells (TekCre-posTlr4loxP/loxP) and TekCre-negTlr4loxP/loxP mice were used. The mice were injected with 1 mg/kg LPS via the tail vein once per day for a total of 4 days. Prior to initiation of LPS injections and approximately 5 h after the last injection, in vivo imaging using fluorescein angiography and spectral-domain optical coherence tomography was performed. Immunohistochemistry, flow cytometry, electroretinography and transmission electron microscopy were utilized to investigate the role of endothelial TLR4 in LPS-induced microglia activation and retinal function.
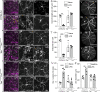
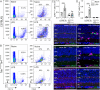
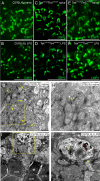
Results: Activation of microglia, infiltration of monocyte-derived macrophages, impaired ribbon synapse organization and retinal dysfunction were observed after the LPS exposure in C57BL/6J and TekCre-negTlr4loxP/loxP mice. None of these effects were observed in the retinas of conditional Tlr4 knockout mice after the LPS challenge.
Conclusions: The findings of the present study suggest that systemic LPS exposure can have detrimental effects in the healthy retina and that TLR4 expressed on endothelial cells is essential for retinal microglia activation and retinal dysfunction upon systemic LPS challenge. This important finding provides new insights into the role of microglia-endothelial cell interaction in inflammatory retinal disease.
Keywords: Endothelial cells; Lipopolysaccharide; Microglia; Monocyte-derived macrophages; Retina; Toll-like receptor 4.
© 2023. The Author(s).
Conflict of interest statement
I.T., Boehringer Ingelheim (F); B.L.S, none; P.E., Novartis Pharma Schweiz (C); M.S.Z., Bayer (F, C), Heidelberg Engineering (S), Novartis (C, I), Boehringer Ingelheim (F); P.M.B., Boehringer Ingelheim (E); D. K., Boehringer Ingelheim (F).
Figures


References
-
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–1608. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

