Bacterial communities in temperate and polar coastal sands are seasonally stable
- PMID: 36739458
- PMCID: PMC9723697
- DOI: 10.1038/s43705-021-00028-w
Bacterial communities in temperate and polar coastal sands are seasonally stable
Abstract
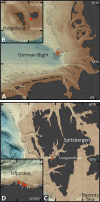
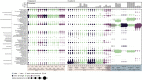
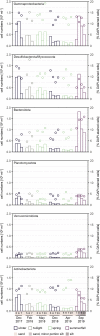
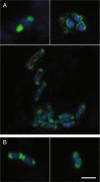
Coastal sands are biocatalytic filters for dissolved and particulate organic matter of marine and terrestrial origin, thus, acting as centers of organic matter transformation. At high temporal resolution, we accessed the variability of benthic bacterial communities over two annual cycles at Helgoland (North Sea), and compared it with seasonality of communities in Isfjorden (Svalbard, 78°N) sediments, where primary production does not occur during winter. Benthic community structure remained stable in both, temperate and polar sediments on the level of cell counts and 16S rRNA-based taxonomy. Actinobacteriota of uncultured Actinomarinales and Microtrichales were a major group, with 8 ± 1% of total reads (Helgoland) and 31 ± 6% (Svalbard). Their high activity (frequency of dividing cells 28%) and in situ cell numbers of >10% of total microbes in Svalbard sediments, suggest Actinomarinales and Microtrichales as key heterotrophs for carbon mineralization. Even though Helgoland and Svalbard sampling sites showed no phytodetritus-driven changes of the benthic bacterial community structure, they harbored significantly different communities (p < 0.0001, r = 0.963). The temporal stability of benthic bacterial communities is in stark contrast to the dynamic succession typical of coastal waters, suggesting that pelagic and benthic bacterial communities respond to phytoplankton productivity very differently.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Boudreau BP, Huettel M, Forster S, Jahnke RA, Middelburg JJ, Boudreau BP, et al. Permeable marine sediments: overturning an old paradigm. Eos Trans AGU. 2001;82:133–6.
-
- Huettel M, Ziebis W, Forster S. Flow-induced uptake of particulate matter in permeable sediments. Limnol Oceanogr. 1996;41:309–22. doi: 10.4319/lo.1996.41.2.0309. - DOI
-
- Huettel M, Rusch A. Transport and degradation of phytoplankton in permeable sediment. Limnol Oceanogr. 2000;45:534–49. doi: 10.4319/lo.2000.45.3.0534. - DOI
-
- Rusch A, Forster S, Huettel M. Bacteria, diatoms and detritus in an intertidal sandflat subject to advective transport across the water-sediment interface. Biogeochemistry. 2001;55:1–27. doi: 10.1023/A:1010687322291. - DOI

