Direct nitrogen, phosphorus and carbon exchanges between Mucoromycotina 'fine root endophyte' fungi and a flowering plant in novel monoxenic cultures
- PMID: 36739554
- PMCID: PMC10952891
- DOI: 10.1111/nph.18630
Direct nitrogen, phosphorus and carbon exchanges between Mucoromycotina 'fine root endophyte' fungi and a flowering plant in novel monoxenic cultures
Abstract
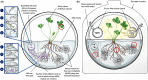
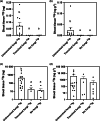
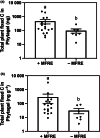
Most plants form mycorrhizal associations with mutualistic soil fungi. Through these partnerships, resources are exchanged including photosynthetically fixed carbon for fungal-acquired nutrients. Recently, it was shown that the diversity of associated fungi is greater than previously assumed, extending to Mucoromycotina fungi. These Mucoromycotina 'fine root endophytes' (MFRE) are widespread and generally co-colonise plant roots together with Glomeromycotina 'coarse' arbuscular mycorrhizal fungi (AMF). Until now, this co-occurrence has hindered the determination of the direct function of MFRE symbiosis. To overcome this major barrier, we developed new techniques for fungal isolation and culture and established the first monoxenic in vitro cultures of MFRE colonising a flowering plant, clover. Using radio- and stable-isotope tracers in these in vitro systems, we measured the transfer of 33 P, 15 N and 14 C between MFRE hyphae and the host plant. Our results provide the first unequivocal evidence that MFRE fungi are nutritional mutualists with a flowering plant by showing that clover gained both 15 N and 33 P tracers directly from fungus in exchange for plant-fixed C in the absence of other micro-organisms. Our findings and methods pave the way for a new era in mycorrhizal research, firmly establishing MFRE as both mycorrhizal and functionally important in terrestrial ecosystems.
Keywords: Mucoromycotina; arbuscular mycorrhizal fungi; carbon; clover; fine root endophytes; flowering plants; soil nutrients; symbiosis.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures

References
-
- Abbott LK. 1982. Comparative anatomy of vesicular arbuscular mycorrhizas formed on subterranean clover. Australian Journal of Botany 30: 485–499.
-
- Albornoz FE, Orchard S, Standish RJ, Dickie IA, Bending G, Hilton S, Lardner T, Foster KJ, Gleeson DB, Bougoure J et al. 2021. Evidence for niche differentiation in the environmental responses of co‐occurring Mucoromycotinian fine root endophytes and Glomeromycotinian arbuscular mycorrhizal fungi. Microbial Ecology 81: 864–873. - PubMed
-
- Ali B. 1969. Occurrence and characteristics of the vesicular arbuscular endophytes of Nardus stricta . Nova Hedwigia 17: 409–425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

