Association of atrial myopathy in mitral valve disease on safety outcomes in left atrial appendage closure
- PMID: 36739561
- PMCID: PMC10241670
- DOI: 10.1007/s00392-022-02151-7
Association of atrial myopathy in mitral valve disease on safety outcomes in left atrial appendage closure
Abstract
Background: Patients undergoing left atrial appendage (LAA) occlusion (LAAO) are multi-morbid, including mitral valve disease (MVD) which is associated with anatomic changes of the left atrium (LA). This study aims to identify how atrial myopathy in MVD influences outcomes in LAAO.
Methods: Atrial myopathy in MVD was defined as LA diameter > 45 mm (♀) and > 48 mm (♂) and existing MVD or history of surgical/interventional treatment. Patients were compared with controls from the prospective, multicentre LAArge registry of LAAO.
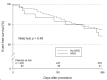
Results: A total of 528 patients (52 MVD, 476 no-MVD) were included. The MVD group was significantly more likely to be older (78.2 years vs 75.9 years, p = 0.036) and female (59.6% vs 37.8%, p = 0.002). Altered LA anatomy was observed in MVD with significantly larger LA diameter (53 mm vs. 48 mm, p < 0.001) and LAA Ostia [at 135° 23.0 mm (20.5, 26.0) vs 20.0 mm (18.0, 23.0), p = 0.002]. Implant success was high with 96.2% and 97.9%, respectively, without differences in severe complications (7.7% vs 4.6%, p = 0.31). One-year mortality (17.8% vs 11.5%, p = 0.19) and a combined outcome of death, stroke, and systemic embolism (20.3% vs 12.4%, p = 0.13) were not different. Independent predictors of the combined outcome were peripheral artery disease (HR 2.41, 95% CI 1.46-3.98, p < 0.001) and chronic kidney disease (HR 3.46, 95% CI 2.02-5.93, p < 0.001) but not MVD and atrial myopathy.
Conclusion: Patients with MVD present with altered LA anatomy with increased LA and LAA diameter. However, procedural success and safety in LAAO are not compromised. One-year mortality is numerically higher in patients with MVD but driven by comorbidities.
Keywords: Atrial fibrillation; Left atrial appendage occlusion; Mitral valve regurgitation; Safety outcomes.
© 2023. The Author(s).
Conflict of interest statement
MH, JS unrestricted grant from Boston Scientific; EL reports grants and personal fees from Abbott Vascular. Outside the submitted work EL received personal fees from Abiomed, Astra Zeneca, Bayer, Edwards Lifesciences, New Valve Technology and Novartis. Other authors report no conflict of interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical