ASC specks exacerbate α‑synuclein pathology via amplifying NLRP3 inflammasome activities
- PMID: 36740674
- PMCID: PMC9899382
- DOI: 10.1186/s12974-023-02709-w
ASC specks exacerbate α‑synuclein pathology via amplifying NLRP3 inflammasome activities
Abstract
Background: Inflammasome activation has a pathogenic role in Parkinson's disease (PD). Up-regulated expressions of inflammasome adaptor apoptosis-associated speck-like protein containing a CARD (ASC) and assembly of ASC specks have been observed in postmortems of human PD brains and experimental PD models. Extracellular ASC specks behave like danger signals and sustain prolonged inflammasome activation. However, the contribution of ASC specks in propagation of inflammasome activation and pathological progression in PD has not been fully established.
Methods: Herein, we used human A53T mutant α-synuclein preformed fibrils (PFFs)-stimulated microglia in vitro and unilateral striatal stereotaxic injection of PFFs-induced mice model of PD in vivo, to investigate the significance of ASC specks in PD pathological progression. Rotarod and open-field tests were performed to measure motor behaviors of indicated mice. Changes in the molecular expression were evaluated by immunofluorescence and immunoblotting (IB). Intracellular knockdown of the ASC in BV2 cells was performed using si-RNA. Microglial and neuronal cells were co-cultured in a trans-well system to determine the effects of ASC knockdown on cytoprotection.
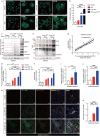
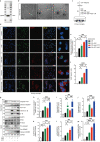
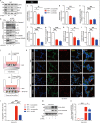
Results: We observed a direct relationship between levels of ASC protein and misfolded α‑synuclein aggregates in PD mice brains. ASC specks amplified NLRP3 inflammasome activation driven by α-synuclein PFFs stimulation, which aggravated reactive microgliosis and accelerated α‑synuclein pathology, dopaminergic neurodegeneration and motor deficits. Endogenous ASC knockdown suppressed microglial inflammasome activation and neuronal α‑synuclein aggregation.
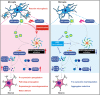
Conclusions: In conclusion, our study elucidated that ASC specks contribute to the propagation of inflammasome activation-associated α‑synuclein pathology in PD, which forms the basis for targeting ASC as a potential therapy for PD.
Keywords: ASC specks; Microglia; NLRP3 inflammasome; Parkinson’s disease; α‑synuclein pathology.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests.
Figures




References
-
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. - PubMed
-
- McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–497. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

