Emerging Roles of Microglia in Blood-Brain Barrier Integrity in Aging and Neurodegeneration
- PMID: 36740799
- PMCID: PMC10964094
- DOI: 10.2174/1570159X21666230203103910
Emerging Roles of Microglia in Blood-Brain Barrier Integrity in Aging and Neurodegeneration
Abstract
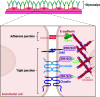
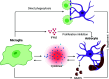
The blood-brain barrier (BBB) is a highly selective interface between the blood and the brain parenchyma. It plays an essential role in maintaining a specialized environment for central nervous system function and homeostasis. The BBB disrupts with age, which contributes to the development of many age-related disorders due to central and peripheral toxic factors or BBB dysfunction. Microglia, the resident innate immune cells of the brain, have recently been explored for their ability to directly and indirectly regulate the integrity of the BBB. This review will focus on the current understanding of the molecular mechanisms utilized by microglia to regulate BBB integrity and how this becomes disrupted in aging and age-associated diseases. We will also discuss the rationale for considering microglia as a therapeutic target to prevent or slow down neurodegeneration.
Keywords: Microglia; aging; blood-brain barrier; neurodegeneration.; neuroinflammation; neurovascular unit.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures



References
-
- Senatorov V.V., Jr, Friedman A.R., Milikovsky D.Z., Ofer J., Saar-Ashkenazy R., Charbash A., Jahan N., Chin G., Mihaly E., Lin J.M., Ramsay H.J., Moghbel A., Preininger M.K., Eddings C.R., Harrison H.V., Patel R., Shen Y., Ghanim H., Sheng H., Veksler R., Sudmant P.H., Becker A., Hart B., Rogawski M.A., Dillin A., Friedman A., Kaufer D. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. 2019;11(521):eaaw8283. doi: 10.1126/scitranslmed.aaw8283. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

