Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study
- PMID: 36740954
- PMCID: PMC11491622
- DOI: 10.1210/clinem/dgad027
Nicotinamide Adenine Dinucleotide Augmentation in Overweight or Obese Middle-Aged and Older Adults: A Physiologic Study
Abstract
Context: Nicotinamide adenine dinucleotide (NAD) levels decline with aging and age-related decline in NAD has been postulated to contribute to age-related diseases.
Objective: We evaluated the safety and physiologic effects of NAD augmentation by administering its precursor, β-nicotinamide mononucleotide (MIB-626, Metro International Biotech, Worcester, MA), in adults at risk for age-related conditions.
Methods: Thirty overweight or obese adults, ≥ 45 years, were randomized in a 2:1 ratio to 2 MIB-626 tablets each containing 500 mg of microcrystalline β-nicotinamide mononucleotide or placebo twice daily for 28 days. Study outcomes included safety; NAD and its metabolome; body weight; liver, muscle, and intra-abdominal fat; insulin sensitivity; blood pressure; lipids; physical performance, and muscle bioenergetics.
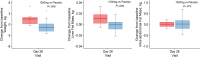
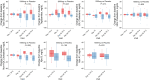
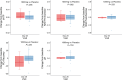
Results: Adverse events were similar between groups. MIB-626 treatment substantially increased circulating concentrations of NAD and its metabolites. Body weight (difference -1.9 [-3.3, -0.5] kg, P = .008); diastolic blood pressure (difference -7.01 [-13.44, -0.59] mmHg, P = .034); total cholesterol (difference -26.89 [-44.34, -9.44] mg/dL, P = .004), low-density lipoprotein (LDL) cholesterol (-18.73 [-31.85, -5.60] mg/dL, P = .007), and nonhigh-density lipoprotein cholesterol decreased significantly more in the MIB-626 group than placebo. Changes in muscle strength, muscle fatigability, aerobic capacity, and stair-climbing power did not differ significantly between groups. Insulin sensitivity and hepatic and intra-abdominal fat did not change in either group.
Conclusions: MIB-626 administration in overweight or obese, middle-aged and older adults safely increased circulating NAD levels, and significantly reduced total LDL and non-HDL cholesterol, body weight, and diastolic blood pressure. These data provide the rationale for larger trials to assess the efficacy of NAD augmentation in improving cardiometabolic outcomes in older adults.
Keywords: blood pressure; geroscience; lipids; metabolic effects; muscle bioenergetics; physical function; β-nicotinamide mononucleotide.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Comment in
-
NAD-elevating Interventions for Cardiometabolic Disease.J Clin Endocrinol Metab. 2023 Aug 18;108(9):e897-e898. doi: 10.1210/clinem/dgad120. J Clin Endocrinol Metab. 2023. PMID: 36869827 No abstract available.
References
-
- Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15(2):241‐246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

