Tetrasodium EDTA for the prevention of urinary catheter infections and blockages
- PMID: 36741142
- PMCID: PMC9832581
- DOI: 10.1039/d2ra06418a
Tetrasodium EDTA for the prevention of urinary catheter infections and blockages
Abstract
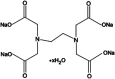
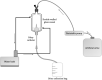
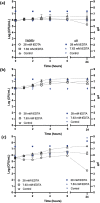
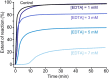
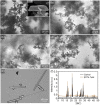
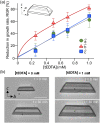
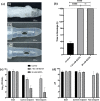
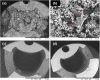
Long-term catheterised individuals are at significant risk of developing catheter-associated urinary tract infections (CAUTIs), with up to 50% of patients experiencing recurrent episodes of catheter encrustation and blockage. Catheter blockage is a result of accumulation of carbonate apatite and struvite formed upon precipitation of ions within urine due to an infection-induced rise in pH. The aim of this study was to investigate the antimicrobial and anti-encrustation activities of tetrasodium ethylenediaminetetraacetic acid (tEDTA) to evaluate its potential efficacy in preventing CAUTIs and catheter blockages. The antimicrobial activity of tEDTA against uropathogens was assessed using time kill assays performed in artificial urine (AU). Crystallisation studies and in vitro bladder model assays were conducted to investigate the effect of tEDTA on struvite crystallisation and catheter blockage. tEDTA displayed bacteriostatic activity against Proteus mirabilis and prevented precipitation of ions in the AU. Crystallisation studies confirmed tEDTA inhibits struvite nucleation and growth via Mg2+ chelation with 7.63 mM tEDTA, equimolar to the concentration of divalent cations in AU, preventing the formation of crystalline deposits and blockage of Foley catheters for ≥168 h. The promising chelating abilities of low tEDTA concentrations could be exploited to inhibit encrustation and blockage of indwelling catheters. The fundamental research presented will inform our future development of an effective tEDTA-eluting catheter coating aimed at preventing catheter encrustation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
None declared.
Figures

Similar articles
-
In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters.J Hosp Infect. 2019 Sep;103(1):55-63. doi: 10.1016/j.jhin.2019.02.012. Epub 2019 Feb 22. J Hosp Infect. 2019. PMID: 30802524
-
Prevention of encrustation and blockage of urinary catheters by Proteus mirabilis via pH-triggered release of bacteriophage.J Mater Chem B. 2017 Jul 21;5(27):5403-5411. doi: 10.1039/c7tb01302g. Epub 2017 Jun 27. J Mater Chem B. 2017. PMID: 32264080
-
Allicin prevents the formation of Proteus-induced urinary crystals and the blockage of catheter in a bladder model in vitro.Microb Pathog. 2019 Jul;132:293-301. doi: 10.1016/j.micpath.2019.05.016. Epub 2019 May 10. Microb Pathog. 2019. PMID: 31082531
-
Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done.J Intern Med. 2014 Aug;276(2):120-9. doi: 10.1111/joim.12220. J Intern Med. 2014. PMID: 24635559 Review.
-
Pathogenesis of Proteus mirabilis in Catheter-Associated Urinary Tract Infections.Urol Int. 2021;105(5-6):354-361. doi: 10.1159/000514097. Epub 2021 Mar 10. Urol Int. 2021. PMID: 33691318 Review.
Cited by
-
Plasma activated water pre-treatment substantially enhances phage activity against Proteus mirabilis biofilms.Biofilm. 2024 Oct 18;8:100230. doi: 10.1016/j.bioflm.2024.100230. eCollection 2024 Dec. Biofilm. 2024. PMID: 39498232 Free PMC article.
References
-
- Irwin N. J. Bryant M. G. McCoy C. P. Trotter J. L. Turner J. Multifunctional, low friction, antimicrobial approach for biomaterial surface enhancement. ACS Appl. Bio Mater. 2020;3(3):1385–1393. - PubMed
-
- Saint S. Chenoweth C. E. Biofilms and catheter-associated urinary tract infections. Infect. Dis. Clin. North Am. 2003;17(2):411–432. - PubMed
-
- Lo E. Nicolle L. Classen D. Arias K. M. Podgorny K. Anderson D. J. et al, Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 2008;29(S1):S41–S50. - PubMed
LinkOut - more resources
Full Text Sources

