Targeting STING: From antiviral immunity to treat osteoporosis
- PMID: 36741390
- PMCID: PMC9891206
- DOI: 10.3389/fimmu.2022.1095577
Targeting STING: From antiviral immunity to treat osteoporosis
Abstract
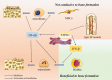
The cGAS-STING signaling pathway can trigger innate immune responses by detecting dsDNA from outside or within the host. In addition, the cGAS-STING signaling pathway has emerged as a critical mediator of the inflammatory response and a new target for inflammatory diseases. STING activation leads to dimerization and translocation to the endoplasmic reticulum Golgi intermediate compartment or Golgi apparatus catalyzed by TBK1, triggers the production of IRF3 and NF-κB and translocates to the nucleus to induce a subsequent interferon response and pro-inflammatory factor production. Osteoporosis is a degenerative bone metabolic disease accompanied by chronic sterile inflammation. Activating the STING/IFN-β signaling pathway can reduce bone resorption by inhibiting osteoclast differentiation. Conversely, activation of STING/NF-κB leads to the formation of osteoporosis by increasing bone resorption and decreasing bone formation. In addition, activation of STING inhibits the generation of type H vessels with the capacity to osteogenesis, thereby inhibiting bone formation. Here, we outline the mechanism of action of STING and its downstream in osteoporosis and discuss the role of targeting STING in the treatment of osteoporosis, thus providing new ideas for the treatment of osteoporosis.
Keywords: IFN-β; NF-κB; STING; osteoporosis; type H vessels.
Copyright © 2023 Gao, Gao, Zhang, Hou, Zhou and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway.J Virol. 2019 May 29;93(12):e02298-18. doi: 10.1128/JVI.02298-18. Print 2019 Jun 15. J Virol. 2019. PMID: 30918080 Free PMC article.
-
Mitochondrial DNA drives noncanonical inflammation activation via cGAS-STING signaling pathway in retinal microvascular endothelial cells.Cell Commun Signal. 2020 Oct 28;18(1):172. doi: 10.1186/s12964-020-00637-3. Cell Commun Signal. 2020. PMID: 33115500 Free PMC article.
-
STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS).Cell Death Dis. 2022 Mar 25;13(3):269. doi: 10.1038/s41419-022-04664-5. Cell Death Dis. 2022. PMID: 35338116 Free PMC article.
-
Relationship between the cGAS-STING and NF-κB pathways-role in neurotoxicity.Biomed Pharmacother. 2024 Jun;175:116698. doi: 10.1016/j.biopha.2024.116698. Epub 2024 May 6. Biomed Pharmacother. 2024. PMID: 38713946 Review.
-
Molecular and spatial mechanisms governing STING signalling.FEBS J. 2021 Oct;288(19):5504-5529. doi: 10.1111/febs.15640. Epub 2020 Dec 11. FEBS J. 2021. PMID: 33237620 Review.
Cited by
-
The role of the cGAS-STING pathway in metabolic diseases.Heliyon. 2024 Jun 14;10(12):e33093. doi: 10.1016/j.heliyon.2024.e33093. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38988528 Free PMC article. Review.
-
COPB1 deficiency triggers osteoporosis with elevated iron stores by inducing osteoblast ferroptosis.J Orthop Translat. 2025 Mar 21;51:312-328. doi: 10.1016/j.jot.2025.01.017. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40206560 Free PMC article.
-
Molecular and Cellular Mechanisms of Osteoporosis.Int J Mol Sci. 2023 Oct 30;24(21):15772. doi: 10.3390/ijms242115772. Int J Mol Sci. 2023. PMID: 37958752 Free PMC article. Review.
-
Integration of bioinformatics and machine learning approaches for the validation of pyrimidine metabolism-related genes and their implications in immunotherapy for osteoporosis.BMC Musculoskelet Disord. 2024 May 22;25(1):402. doi: 10.1186/s12891-024-07512-z. BMC Musculoskelet Disord. 2024. PMID: 38778304 Free PMC article.
-
Bioinformatics and machine learning were used to validate glutamine metabolism-related genes and immunotherapy in osteoporosis patients.J Orthop Surg Res. 2023 Sep 14;18(1):685. doi: 10.1186/s13018-023-04152-2. J Orthop Surg Res. 2023. PMID: 37710308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

