Mycobacterium avium subsp. paratuberculosis antigens induce cellular immune responses in cattle without causing reactivity to tuberculin in the tuberculosis skin test
- PMID: 36741398
- PMCID: PMC9889921
- DOI: 10.3389/fimmu.2022.1087015
Mycobacterium avium subsp. paratuberculosis antigens induce cellular immune responses in cattle without causing reactivity to tuberculin in the tuberculosis skin test
Abstract
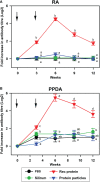
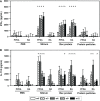
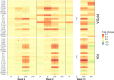
Mycobacterium avium subspecies paratuberculosis (MAP) causes chronic progressive granulomatous enteritis leading to diarrhea, weight-loss, and eventual death in ruminants. Commercially available vaccine provides only partial protection against MAP infection and can interfere with the use of current diagnostic tests for bovine tuberculosis in cattle. Here, we characterized immune responses in calves to vaccines containing four truncated MAP antigens as a fusion (Ag85A202-347-SOD1-72-Ag85B173-330-74F1-148+669-786), either displayed on protein particles, or expressed as a soluble recombinant MAP (rMAP) fusion protein as well as to commercially available Silirum® vaccine. The rMAP fusion protein elicited the strongest antigen-specific antibody responses to both PPDA and recombinant antigen and strong and long-lasting T-cell immune responses to these antigens, as indicated by increased production of IFN-γ and IL-17A in antigen-stimulated whole blood cultures. The MAP fusion protein particle vaccine induced minimal antibody responses and weak IFN-γ responses but stimulated IL-17A responses to recombinant antigen. The immune response profile of Silirum® vaccine was characterized by weak antibodies and strong IFN-γ and IL-17A responses to PPDA. Transcription analysis on antigen-stimulated leukocytes from cattle vaccinated with rMAP fusion protein showed differential expression of several immune response genes and genes involved in costimulatory signaling, TLR4, TLR2, PTX3, PTGS2, PD-L1, IL1B, IL2, IL6, IL12B, IL17A, IL22, IFNG, CD40, and CD86. Moreover, the expression of several genes of immune pathways correlated with cellular immune responses in the rMAP fusion protein vaccinated group. These genes have key roles in pathways of mycobacterial immunity, including autophagy, manipulation of macrophage-mediated killing, Th17- and regulatory T cells- (Treg) mediated responses. Calves vaccinated with either the rMAP fusion protein or MAP fusion protein particle vaccine did not induce reactivity to PPDA and PPDB in a comparative cervical skin test, whereas Silirum® induced reactivity to these tuberculins in most of the vaccinated animals. Overall, our results suggest that a combination of recombinant MAP antigens in the form of a soluble fusion protein vaccine are capable of inducing strong antigen-specific humoral and a balanced Th1/Th17-cell immune response. These findings, together with the absence of reactivity to tuberculin, suggest this subunit vaccine could provide protective immunity against intracellular MAP infection in cattle without compromising the use of current bovine tuberculosis surveillance test.
Keywords: IFN-γ; IL-17; Johne’s disease; Mycobacterium avium subspecies paratuberculosis; gene expression; nanostring; recombinant MAP fusion protein particle vaccine; tuberculin skin test.
Copyright © 2023 Gupta, Wilson, Maclean, Rehm, Heiser, Buddle and Wedlock.
Conflict of interest statement
Authors SG, TW, PM, AH, BB and DW were employed by company AgResearch. The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

