SLC46A3 is a lysosomal proton-coupled steroid conjugate and bile acid transporter involved in transport of active catabolites of T-DM1
- PMID: 36741448
- PMCID: PMC9896951
- DOI: 10.1093/pnasnexus/pgac063
SLC46A3 is a lysosomal proton-coupled steroid conjugate and bile acid transporter involved in transport of active catabolites of T-DM1
Abstract
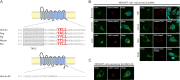
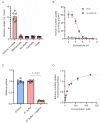
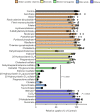
Antibody-drug conjugates (ADCs) represent a new class of cancer therapeutics that enable targeted delivery of cytotoxic drugs to cancer cells. Although clinical efficacy has been demonstrated for ADC therapies, resistance to these conjugates may occur. Recently, SLC46A3, a lysosomal membrane protein, was revealed to regulate the efficacy of trastuzumab emtansine (T-DM1), a noncleavable ADC that has been widely used for treating breast cancer. However, the role of SLC46A3 in mediating T-DM1 cytotoxicity remains unclear. In this study, we discovered the function of SLC46A3 as a novel proton-coupled steroid conjugate and bile acid transporter. SLC46A3 preferentially recognized lipophilic steroid conjugates and bile acids as endogenous substrates. In addition, we found that SLC46A3 directly transports Lys-SMCC-DM1, a major catabolite of T-DM1, and potent SLC46A3 inhibitors attenuate the cytotoxic effects of T-DM1, suggesting a role in the escape of Lys-SMCC-DM1 from the lysosome into the cytoplasm. Our findings reveal the molecular mechanism by which T-DM1 kills cancer cells and may contribute to the rational development of ADCs that target SLC46A3.
Keywords: SLC46A3; and trastuzumab emtansine; antibody–drug conjugate; bile acid; steroid conjugate.
© The Author(s) 2022. Published by Oxford University Press on behalf of the National Academy of Sciences.
Figures

References
-
- Chau CH, Steeg PS, Figg WD. 2019. Antibody-drug conjugates for cancer. Lancet. 394:793–804. - PubMed
-
- Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. 2020. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 18:3–19. - PubMed
-
- Coats S, et al. . 2019. Antibody-drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 25:5441–5448. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

