D139N mutation of PsbP enhances the oxygen-evolving activity of photosystem II through stabilized binding of a chloride ion
- PMID: 36741451
- PMCID: PMC9896922
- DOI: 10.1093/pnasnexus/pgac136
D139N mutation of PsbP enhances the oxygen-evolving activity of photosystem II through stabilized binding of a chloride ion
Abstract
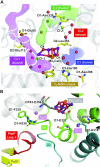
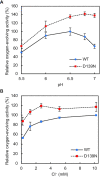
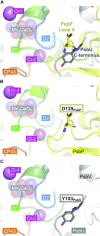
Photosystem II (PSII) is a multisubunit membrane protein complex that catalyzes light-driven oxidation of water to molecular oxygen. The chloride ion (Cl-) has long been known as an essential cofactor for oxygen evolution by PSII, and two Cl- ions (Cl-1 and Cl-2) have been found to specifically bind near the Mn4CaO5 cluster within the oxygen-evolving center (OEC). However, despite intensive studies on these Cl- ions, little is known about the function of Cl-2, the Cl- ion that is associated with the backbone nitrogens of D1-Asn338, D1-Phe339, and CP43-Glu354. In green plant PSII, the membrane extrinsic subunits-PsbP and PsbQ-are responsible for Cl- retention within the OEC. The Loop 4 region of PsbP, consisting of highly conserved residues Thr135-Gly142, is inserted close to Cl-2, but its importance has not been examined to date. Here, we investigated the importance of PsbP-Loop 4 using spinach PSII membranes reconstituted with spinach PsbP proteins harboring mutations in this region. Mutations in PsbP-Loop 4 had remarkable effects on the rate of oxygen evolution by PSII. Moreover, we found that a specific mutation, PsbP-D139N, significantly enhances the oxygen-evolving activity in the absence of PsbQ, but not significantly in its presence. The D139N mutation increased the Cl- retention ability of PsbP and induced a unique structural change in the OEC, as indicated by light-induced Fourier transform infrared (FTIR) difference spectroscopy and theoretical calculations. Our findings provide insight into the functional significance of Cl-2 in the water-oxidizing reaction of PSII.
Keywords: chloride ions; membrane-extrinsic proteins; oxygen evolution; photosynthesis.
© The Author(s) 2022. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures




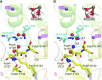
Similar articles
-
Binding and functions of the two chloride ions in the oxygen-evolving center of photosystem II.Photosynth Res. 2022 Sep;153(3):135-156. doi: 10.1007/s11120-022-00921-y. Epub 2022 Jun 13. Photosynth Res. 2022. PMID: 35698013 Review.
-
The PsbQ protein stabilizes the functional binding of the PsbP protein to photosystem II in higher plants.Biochim Biophys Acta. 2012 Aug;1817(8):1346-51. doi: 10.1016/j.bbabio.2012.01.009. Epub 2012 Jan 25. Biochim Biophys Acta. 2012. PMID: 22306528
-
FTIR evidence that the PsbP extrinsic protein induces protein conformational changes around the oxygen-evolving Mn cluster in photosystem II.Biochemistry. 2009 Jul 14;48(27):6318-25. doi: 10.1021/bi9006308. Biochemistry. 2009. PMID: 19492796
-
Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II.Front Plant Sci. 2016 Feb 5;7:84. doi: 10.3389/fpls.2016.00084. eCollection 2016. Front Plant Sci. 2016. PMID: 26904056 Free PMC article. Review.
-
Cross-linking evidence for multiple interactions of the PsbP and PsbQ proteins in a higher plant photosystem II supercomplex.J Biol Chem. 2014 Jul 18;289(29):20150-7. doi: 10.1074/jbc.M114.574822. Epub 2014 Jun 9. J Biol Chem. 2014. PMID: 24914208 Free PMC article.
Cited by
-
Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits.Int J Mol Sci. 2025 Mar 24;26(7):2922. doi: 10.3390/ijms26072922. Int J Mol Sci. 2025. PMID: 40243522 Free PMC article.
-
The lumenal domain of Cyt b559 interacting with extrinsic subunits is crucial for accumulation of functional photosystem II.Photosynth Res. 2025 Jun 10;163(3):33. doi: 10.1007/s11120-025-01157-2. Photosynth Res. 2025. PMID: 40493130
References
-
- Debus RJ. 1992. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta Bioenerg. 1102:269–352. - PubMed
-
- Umena Y, Kawakami K, Shen J-R, Kamiya N. 2011. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 473:55–60. - PubMed
-
- Suga M, et al. 2015. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature. 517:99–103. - PubMed
-
- Kok B, Forbush B, McGloin M. 1970. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 11:457–475. - PubMed
-
- Wei X, et al. 2016. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nature. 534:69–74. - PubMed

