Segregation of D1 and D2 dopamine receptors in the striatal direct and indirect pathways: An historical perspective
- PMID: 36741471
- PMCID: PMC9892636
- DOI: 10.3389/fnsyn.2022.1002960
Segregation of D1 and D2 dopamine receptors in the striatal direct and indirect pathways: An historical perspective
Abstract
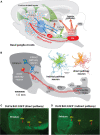
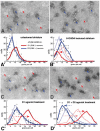
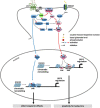
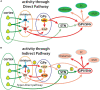
The direct and indirect striatal pathways form a cornerstone of the circuits of the basal ganglia. Dopamine has opponent affects on the function of these pathways due to the segregation of the D1- and D2-dopamine receptors in the spiny projection neurons giving rise to the direct and indirect pathways. An historical perspective is provided on the discovery of dopamine receptor segregation leading to models of how the direct and indirect affect motor behavior.
Keywords: Parkinson’s disease; basal ganglia; dopamine; motor function; striatum.
Copyright © 2023 This work is authored by Charles R. Gerfen on behalf of the U.S. Government and as regards Dr. Gerfen, and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anderson M. E., Inase M., Buford J., Turner R. S., Mano N., Hamada I., et al. (1993). “Movement and preparatory activity of neurons in pallidal-receiv ing areas of the monkey thalamus,” in Role of the cerebellum and basal ganglia in voluntary movement, (Amsterdam: Elsevier Science Publishers; ).
Publication types
LinkOut - more resources
Full Text Sources

