Resistance to single-agent chemotherapy in low-risk gestational trophoblastic neoplasia
- PMID: 36741497
- PMCID: PMC9878912
- DOI: 10.22088/cjim.14.1.47
Resistance to single-agent chemotherapy in low-risk gestational trophoblastic neoplasia
Abstract
Background: Methotrexate (MTX) and actinomycin D (ActD) have been used as first-line chemotherapy agents in the treatment of low-risk gestational trophoblastic neoplasia (GTN). Although low-risk GTN is considered a curable disease, its reported primary remission rates of 49 to 93% reflect the difficulties of treatment and different factors influencing it. Hence, this study aimed to determine the remission rates and related factors of single-agent chemotherapy resistance in low-risk GTN patients.
Methods: This retrospective study included patients with diagnosed low-risk GTN who received either MTX once a week (IM, 30mg/m2) or ActD once every two weeks (pulsed IV, 1.25mg/m2). Then, the patients were followed-up until complete remission or single-agent treatment failure to assess resistance rate and related factors.
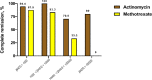
Results: Eighty-four patients were included in the study (18 patients were receiving MTX and 66 patients were receiving ActD). 85.7% of all participants achieved complete remission after first-line chemotherapy (72.2% in MTX vs 89.4% in ActD). There was a significant association for higher tumor size (P=0.046), the occurrence of metastasis (P=0.019), and pretreatment β-HCG levels (P=0.005) with resistance to treatment.
Conclusion: This study demonstrated higher tumor size, the occurrence of metastasis, and pretreatment β-HCG levels have been associated with increased resistance to first-line chemotherapy agents.
Keywords: Dactinomycin; Gestational trophoblastic neoplasia; Methotrexate; Single-agent chemotherapy; Treatment failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Effectiveness and toxicity of second-line actinomycin D in patients with methotrexate-resistant postmolar low-risk gestational trophoblastic neoplasia.Gynecol Oncol. 2020 May;157(2):372-378. doi: 10.1016/j.ygyno.2020.02.001. Epub 2020 Feb 7. Gynecol Oncol. 2020. PMID: 32037196
-
Increasing the human chorionic gonadotrophin cut-off to ≤1000 IU/l for starting actinomycin D in post-molar gestational trophoblastic neoplasia developing resistance to methotrexate spares more women multi-agent chemotherapy.ESMO Open. 2021 Jun;6(3):100110. doi: 10.1016/j.esmoop.2021.100110. Epub 2021 Apr 10. ESMO Open. 2021. PMID: 33845362 Free PMC article.
-
Efficacy analysis of single-agent carboplatin AUC4 2-weekly as second-line therapy for methotrexate-resistant (MTX-R) low risk gestational trophoblastic neoplasia (GTN).Gynecol Oncol. 2023 Aug;175:66-71. doi: 10.1016/j.ygyno.2023.05.072. Epub 2023 Jun 14. Gynecol Oncol. 2023. PMID: 37327541
-
Treatment of low-risk gestational trophoblastic neoplasia.Best Pract Res Clin Obstet Gynaecol. 2021 Jul;74:67-80. doi: 10.1016/j.bpobgyn.2021.01.006. Epub 2021 Feb 2. Best Pract Res Clin Obstet Gynaecol. 2021. PMID: 33741258 Review.
-
Real-world data of 14 cases of brain metastases from gestational trophoblastic neoplasia and a literature review.Arch Gynecol Obstet. 2022 Apr;305(4):929-935. doi: 10.1007/s00404-021-06238-w. Epub 2021 Sep 20. Arch Gynecol Obstet. 2022. PMID: 34542678 Review.
Cited by
-
Effectiveness and Efficiency Comparison of One-Day vs Eight-Day Methotrexate Protocols in Managing Low-Risk Gestational Trophoblastic Neoplasia.Int J Womens Health. 2024 Dec 3;16:2077-2085. doi: 10.2147/IJWH.S486620. eCollection 2024. Int J Womens Health. 2024. PMID: 39649071 Free PMC article.
References
-
- Goldstein DP, Berkowitz RS, Horowitz NS. Optimal management of low-risk gestational trophoblastic neoplasia. Expert Rev Anticancer Ther. 2015;15:1293–304. - PubMed
-
- Gockley AA, Joseph NT, Melamed A, et al. Effect of race/ethnicity on clinical presentation and risk of gestational trophoblastic neoplasia in patients with complete and partial molar pregnancy at a tertiary care referral center. Am J Obstet Gynecol. 2016;215:334.e1–6. - PubMed
LinkOut - more resources
Full Text Sources