Metal complexes for catalytic and photocatalytic reactions in living cells and organisms
- PMID: 36741514
- PMCID: PMC9848159
- DOI: 10.1039/d2sc05672k
Metal complexes for catalytic and photocatalytic reactions in living cells and organisms
Abstract
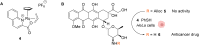
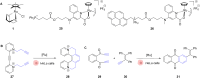
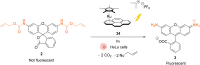
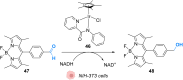
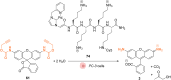
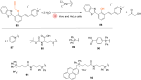
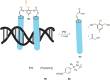
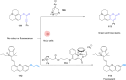
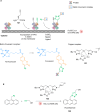
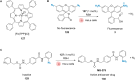
The development of organometallic catalysis has greatly expanded the synthetic chemist toolbox compared to only exploiting "classical" organic chemistry. Although more widely used in organic solvents, metal-based catalysts have also emerged as efficient tools for developing organic transformations in water, thus paving the way for further development of bio-compatible reactions. However, performing metal-catalysed reactions within living cells or organisms induces additional constraints to the design of reactions and catalysts. In particular, metal complexes must exhibit good efficiency in complex aqueous media at low concentrations, good cell specificity, good cellular uptake and low toxicity. In this review, we focus on the presentation of discrete metal complexes that catalyse or photocatalyse reactions within living cells or living organisms. We describe the different reaction designs that have proved to be successful under these conditions, which involve very few metals (Ir, Pd, Ru, Pt, Cu, Au, and Fe) and range from in cellulo deprotection/decaging/activation of fluorophores, drugs, proteins and DNA to in cellulo synthesis of active molecules, and protein and organelle labelling. We also present developments in bio-compatible photo-activatable catalysts, which represent a very recent emerging area of research and some prospects in the field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



































References
Publication types
LinkOut - more resources
Full Text Sources

