Chemistry-mechanics-geometry coupling in positive electrode materials: a scale-bridging perspective for mitigating degradation in lithium-ion batteries through materials design
- PMID: 36741524
- PMCID: PMC9848157
- DOI: 10.1039/d2sc04157j
Chemistry-mechanics-geometry coupling in positive electrode materials: a scale-bridging perspective for mitigating degradation in lithium-ion batteries through materials design
Abstract
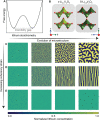
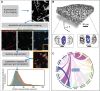
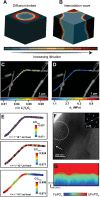
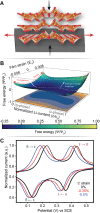
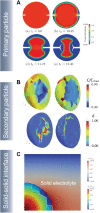
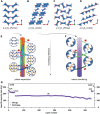
Despite their rapid emergence as the dominant paradigm for electrochemical energy storage, the full promise of lithium-ion batteries is yet to be fully realized, partly because of challenges in adequately resolving common degradation mechanisms. Positive electrodes of Li-ion batteries store ions in interstitial sites based on redox reactions throughout their interior volume. However, variations in the local concentration of inserted Li-ions and inhomogeneous intercalation-induced structural transformations beget substantial stress. Such stress can accumulate and ultimately engender substantial delamination and transgranular/intergranular fracture in typically brittle oxide materials upon continuous electrochemical cycling. This perspective highlights the coupling between electrochemistry, mechanics, and geometry spanning key electrochemical processes: surface reaction, solid-state diffusion, and phase nucleation/transformation in intercalating positive electrodes. In particular, we highlight recent findings on tunable material design parameters that can be used to modulate the kinetics and thermodynamics of intercalation phenomena, spanning the range from atomistic and crystallographic materials design principles (based on alloying, polymorphism, and pre-intercalation) to emergent mesoscale structuring of electrode architectures (through control of crystallite dimensions and geometry, curvature, and external strain). This framework enables intercalation chemistry design principles to be mapped to degradation phenomena based on consideration of mechanics coupling across decades of length scales. Scale-bridging characterization and modeling, along with materials design, holds promise for deciphering mechanistic understanding, modulating multiphysics couplings, and devising actionable strategies to substantially modify intercalation phase diagrams in a manner that unlocks greater useable capacity and enables alleviation of chemo-mechanical degradation mechanisms.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures








Similar articles
-
Equilibria and Rate Phenomena from Atomistic to Mesoscale: Simulation Studies of Magnetite.Acc Chem Res. 2018 Mar 20;51(3):583-590. doi: 10.1021/acs.accounts.7b00531. Epub 2018 Mar 2. Acc Chem Res. 2018. PMID: 29498267
-
Electrode Degradation in Lithium-Ion Batteries.ACS Nano. 2020 Feb 25;14(2):1243-1295. doi: 10.1021/acsnano.9b04365. Epub 2020 Feb 4. ACS Nano. 2020. PMID: 31895532
-
Design Strategies of Spinel Oxide Frameworks Enabling Reversible Mg-Ion Intercalation.Acc Chem Res. 2024 Jan 2;57(1):1-9. doi: 10.1021/acs.accounts.3c00282. Epub 2023 Dec 19. Acc Chem Res. 2024. PMID: 38113116
-
Strategies to alleviate distortive phase transformations in Li-ion intercalation reactions: an example with vanadium pentoxide.Nanoscale. 2024 May 23;16(20):9710-9727. doi: 10.1039/d3nr06138h. Nanoscale. 2024. PMID: 38682562 Review.
-
Solvated Ion Intercalation in Graphite: Sodium and Beyond.Front Chem. 2020 May 21;8:432. doi: 10.3389/fchem.2020.00432. eCollection 2020. Front Chem. 2020. PMID: 32509735 Free PMC article. Review.
Cited by
-
Chemomechanical damage prediction from phase-field simulation video sequences using a deep-learning-based methodology.iScience. 2024 Aug 26;27(9):110822. doi: 10.1016/j.isci.2024.110822. eCollection 2024 Sep 20. iScience. 2024. PMID: 39310766 Free PMC article.
-
Interplay Between Stereochemically Active Lone Pair Repulsions, Sigma Hole Interactions, and Delocalized Redox Processes in Topochemical Fluoride-Ion Insertion.Angew Chem Int Ed Engl. 2025 Aug 11;64(33):e202507650. doi: 10.1002/anie.202507650. Epub 2025 Jun 23. Angew Chem Int Ed Engl. 2025. PMID: 40526830 Free PMC article.
-
Interplay between Cation "Coloring" and Stereochemically Active Lone Pairs in AgBiS2 Thin Films.Inorg Chem. 2025 May 26;64(20):10097-10105. doi: 10.1021/acs.inorgchem.5c00710. Epub 2025 May 13. Inorg Chem. 2025. PMID: 40356472 Free PMC article.
-
Nanotechnology solutions for the climate crisis.Nat Nanotechnol. 2024 Oct;19(10):1422-1426. doi: 10.1038/s41565-024-01772-5. Nat Nanotechnol. 2024. PMID: 39385059
References
-
- Cano Z. P. Banham D. Ye S. Hintennach A. Lu J. Fowler M. Chen Z. Nat. Energy. 2018 3;4(3):279–289.
-
- Park N. Y. Ryu H. H. Kuo L. Y. Kaghazchi P. Yoon C. S. Sun Y. K. ACS Energy Lett. 2021;6:4195–4202. doi: 10.1021/acsenergylett.1c02281. - DOI
-
- Masias A. Marcicki J. Paxton W. A. ACS Energy Lett. 2021;6:621–630. doi: 10.1021/acsenergylett.0c02584. - DOI
-
- Kim T. Song W. Son D. Y. Ono L. K. Qi Y. J. Mater. Chem. A. 2019;7:2942–2964. doi: 10.1039/C8TA10513H. - DOI
Publication types
LinkOut - more resources
Full Text Sources

