Development of a dual-component infection-resistant arterial replacement for small-caliber reconstructions: A proof-of-concept study
- PMID: 36741762
- PMCID: PMC9889865
- DOI: 10.3389/fbioe.2023.957458
Development of a dual-component infection-resistant arterial replacement for small-caliber reconstructions: A proof-of-concept study
Abstract
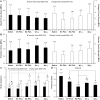
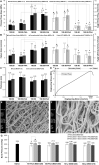
Introduction: Synthetic vascular grafts perform poorly in small-caliber (<6mm) anastomoses, due to intimal hyperplasia and thrombosis, whereas homografts are associated with limited availability and immunogenicity, and bioprostheses are prone to aneurysmal degeneration and calcification. Infection is another important limitation with vascular grafting. This study developed a dual-component graft for small-caliber reconstructions, comprising a decellularized tibial artery scaffold and an antibiotic-releasing, electrospun polycaprolactone (PCL)/polyethylene glycol (PEG) blend sleeve. Methods: The study investigated the effect of nucleases, as part of the decellularization technique, and two sterilization methods (peracetic acid and γ-irradiation), on the scaffold's biological and biomechanical integrity. It also investigated the effect of different PCL/PEG ratios on the antimicrobial, biological and biomechanical properties of the sleeves. Tibial arteries were decellularized using Triton X-100 and sodium-dodecyl-sulfate. Results: The scaffolds retained the general native histoarchitecture and biomechanics but were depleted of glycosaminoglycans. Sterilization with peracetic acid depleted collagen IV and produced ultrastructural changes in the collagen and elastic fibers. The two PCL/PEG ratios used (150:50 and 100:50) demonstrated differences in the structural, biomechanical and antimicrobial properties of the sleeves. Differences in the antimicrobial activity were also found between sleeves fabricated with antibiotics supplemented in the electrospinning solution, and sleeves soaked in antibiotics. Discussion: The study demonstrated the feasibility of fabricating a dual-component small-caliber graft, comprising a scaffold with sufficient biological and biomechanical functionality, and an electrospun PCL/PEG sleeve with tailored biomechanics and antibiotic release.
Keywords: antimicrobial activity; biomechanics; decellularized scaffold; polymeric sleeve; small-caliber vascular graft; tibial artery.
Copyright © 2023 Zia, Djalali-Cuevas, Pflaum, Hegermann, Dipresa, Kalozoumis, Kouvaka, Burgwitz, Andriopoulou, Repanas, Will, Grote, Schrimpf, Toumpaniari, Mueller, Glasmacher, Haverich, Morticelli and Korossis.
Conflict of interest statement
FW was employed by LLS ROWIAK LaserLabSolutions GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bisdas T., Beckmann E., Marsch G., Burgwitz K., Wilhelmi M., Kuehn C., et al. (2012). Prevention of vascular graft infections with antibiotic graft impregnation prior to implantation: In vitro comparison between daptomycin, rifampin and nebacetin. Eur. J. Vasc. Endovasc. Surg. 43, 448–456. 10.1016/j.ejvs.2011.12.029 - DOI - PubMed
-
- Böer U., Spengler C., Jonigk D., Klingenberg M., Schrimpf C., Lützner S., et al. (2013). Coating decellularized equine carotid arteries with ccn1 improves cellular repopulation, local biocompatibility, and immune response in sheep. Tissue Eng. Part a. 19, 1829–1842. 10.1089/ten.tea.2012.0558 - DOI - PubMed
LinkOut - more resources
Full Text Sources

