The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial
- PMID: 36742008
- PMCID: PMC9889936
- DOI: 10.3389/fnut.2022.1023997
The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial
Abstract
Background: Curcumin (CUR) and quercetin (QUE), two natural polyphenols, possess diverse biological activities including broad-spectrum antiviral, antioxidant, and immunomodulatory effects. Both CUR and QUE have shown inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in in vitro assays.
Objective: In the present study we aimed to assess the possible treatment benefits of a combined curcumin and quercetin (CUR-QUE) oral supplement, alongside standard of care (SOC), in the early-stage COVID-19 infection.
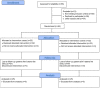
Methods: This was an exploratory, pragmatic, open-label, randomized controlled clinical trial, conducted at the Department of Pathology, Liaquat University of Medical and Health Sciences, Jamshoro, PK. The study compared the treatment effect of an oral CUR-QUE supplement plus SOC vs. SOC alone, in the early-stage/mild to moderately symptomatic COVID-19 outpatients. Patients were randomized in a 1:1 ratio to CUR-QUE (n = 25) and control (n = 25) treatment groups. The CUR-QUE supplementation consisted of a daily intake of 168 mg curcumin and 260 mg quercetin, as two soft capsules, to be taken twice a day at home for 14 days.
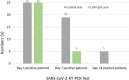
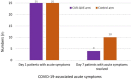
Results: After one-week of treatment, most of the patients in the CUR-QUE group showed an expedited clearance of the viral infection i.e., 18 (72.0%) vs. 6 (24.0%) patients in the control group tested negative for SARS-CoV-2 in the nasal-oropharyngeal swab reverse transcription-polymerase chain reaction (RT-PCR) analysis (p = 0.0002). In addition, COVID-19-associated acute symptoms were also speedily resolved in the CUR-QUE treated patients, i.e., 10 (40.0%) vs. 4 (16.0%) patients in the control group (p = 0.061). The CUR-QUE supplementation therapy was well-tolerated by all 25 patients and no treatment-emergent effects or serious adverse events were reported.
Conclusion: The results revealed in this exploratory study suggest a possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19. It is proposed that the two agents possibly acting in synergy, interfere the SARS-CoV-2 replication, and thus help a speedy recovery in the early-stage of COVID-19. Further research is highly encouraged.
Clinical trial registration: Clinicaltrials.gov, Identifier NCT04603690.
Keywords: COVID-19; SARS-CoV-2; curcumin; polyphenols; quercetin.
Copyright © 2023 Ujjan, Khan, Nigar, Ahmed, Ahmad and Khan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The double-edged sword of nutraceuticals: comprehensive review of protective agents and their hidden risks.Front Nutr. 2025 Mar 27;12:1524627. doi: 10.3389/fnut.2025.1524627. eCollection 2025. Front Nutr. 2025. PMID: 40212723 Free PMC article. Review.
-
Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial.Front Pharmacol. 2022 Jun 7;13:898062. doi: 10.3389/fphar.2022.898062. eCollection 2022. Front Pharmacol. 2022. PMID: 35747751 Free PMC article.
-
Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial.Front Pharmacol. 2023 Jan 13;13:1096853. doi: 10.3389/fphar.2022.1096853. eCollection 2022. Front Pharmacol. 2023. PMID: 36712674 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
Cited by
-
The double-edged sword of nutraceuticals: comprehensive review of protective agents and their hidden risks.Front Nutr. 2025 Mar 27;12:1524627. doi: 10.3389/fnut.2025.1524627. eCollection 2025. Front Nutr. 2025. PMID: 40212723 Free PMC article. Review.
-
Therapeutic implications of quercetin and its derived-products in COVID-19 protection and prophylactic.Heliyon. 2024 Apr 30;10(9):e30080. doi: 10.1016/j.heliyon.2024.e30080. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765079 Free PMC article. Review.
-
COVID-19-Associated Sepsis: Potential Role of Phytochemicals as Functional Foods and Nutraceuticals.Int J Mol Sci. 2024 Aug 3;25(15):8481. doi: 10.3390/ijms25158481. Int J Mol Sci. 2024. PMID: 39126050 Free PMC article. Review.
-
Understanding chronic inflammation: couplings between cytokines, ROS, NO, Cai 2+, HIF-1α, Nrf2 and autophagy.Front Immunol. 2025 Apr 8;16:1558263. doi: 10.3389/fimmu.2025.1558263. eCollection 2025. Front Immunol. 2025. PMID: 40264757 Free PMC article. Review.
-
Relationships between the Planetary Health Diet Index, its food groups, and polygenic risk of obesity in the CARTaGENE cohort.Nutr Metab (Lond). 2024 Dec 31;21(1):116. doi: 10.1186/s12986-024-00890-0. Nutr Metab (Lond). 2024. PMID: 39741271 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

