Genetic removal of synaptic Zn2+ impairs cognition, alters neurotrophic signaling and induces neuronal hyperactivity
- PMID: 36742045
- PMCID: PMC9895830
- DOI: 10.3389/fneur.2022.882635
Genetic removal of synaptic Zn2+ impairs cognition, alters neurotrophic signaling and induces neuronal hyperactivity
Abstract
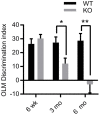
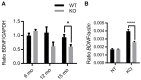
Vesicular Zn2+ (zinc) is released at synapses and has been demonstrated to modulate neuronal responses. However, mechanisms through which dysregulation of zinc homeostasis may potentiate neuronal dysfunction and neurodegeneration are not well-understood. We previously reported that accumulation of soluble amyloid beta oligomers (AβO) at synapses correlates with synaptic loss and that AβO localization at synapses is regulated by synaptic activity and enhanced by the release of vesicular Zn2+ in the hippocampus, a brain region that deteriorates early in Alzheimer's disease (AD). Significantly, drugs regulating zinc homeostasis inhibit AβO accumulation and improve cognition in mouse models of AD. We used both sexes of a transgenic mouse model lacking synaptic Zn2+ (ZnT3KO) that develops AD-like cognitive impairment and neurodegeneration to study the effects of disruption of Zn2+ modulation of neurotransmission in cognition, protein expression and activation, and neuronal excitability. Here we report that the genetic removal of synaptic Zn2+ results in progressive impairment of hippocampal-dependent memory, reduces activity-dependent increase in Erk phosphorylation and BDNF mRNA, alters regulation of Erk activation by NMDAR subunits, increases neuronal spiking, and induces biochemical and morphological alterations consistent with increasing epileptiform activity and neurodegeneration as ZnT3KO mice age. Our study shows that disruption of synaptic Zn2+ triggers neurodegenerative processes and is a potential pathway through which AβO trigger altered expression of neurotrophic proteins, along with reduced hippocampal synaptic density and degenerating neurons, neuronal spiking activity, and cognitive impairment and supports efforts to develop therapeutics to preserve synaptic zinc homeostasis in the brain as potential treatments for AD.
Keywords: Alzheimer's disease (AD); ZnT3; hippocampus; neurodegeneration; neuronal hyperactivity; neurotrophic signaling; synaptic zinc; zinc transporter.
Copyright © 2023 Vogler, Mahavongtrakul, Sarkan, Bohannan, Catuara-Solarz and Busciglio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

