The effect of l-carnitine supplementation on mortality and clinical outcomes in ventilator-dependent critically ill patients with obesity and COVID-19: Protocol for a randomized double-blind placebo-controlled trial
- PMID: 36742110
- PMCID: PMC9886566
- DOI: 10.1016/j.conctc.2023.101082
The effect of l-carnitine supplementation on mortality and clinical outcomes in ventilator-dependent critically ill patients with obesity and COVID-19: Protocol for a randomized double-blind placebo-controlled trial
Abstract
Background: Coronavirus disease 2019 (COVID-19) still remains a pandemic accounting for at least 15% of intensive care unit (ICU) admissions. Recently, it has been observed that l-carnitine levels, which play an important role in fatty acid metabolism, have an inverse association with the severity of COVID-19 and its complications, hence a potential role for supplementing with this nutraceutical has been suggested. The current protocol describes a trial aiming to an evaluation of the effect of l-carnitine intervention on mortality and clinical outcomes in ICU-admitted patients with COVID-19.
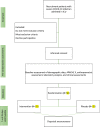
Methods: This parallel-group, randomized, placebo-controlled, and double-blind clinical trial involves 50 participants and will be performed at the ICU of Artesh (AJA) Hospital, Mashhad, IRAN. Eligible participants will be randomized into two groups: 1) the intervention group will receive 1000 mg l-carnitine capsules 3 times a day, and 2) the placebo group will receive 1000 mg placebo capsules 3 times a day. Assessments will be performed at baseline, 7 and 28 days after study initiation. The primary outcome includes changes in serum levels of C-reactive protein (CRP). Secondary outcomes include the length of stay in the ICU, ICU mortality, hospital mortality, 28-day mortality, duration of mechanical ventilation (MV), and the neutrophil-lymphocyte ratio (NLR).
Conclusion: Based on previous evidence, l-carnitine may reduce inflammation and oxidation stress and improve respiratory function. However, the effects of l-carnitine on ventilator-dependent COVID-19 critically ill patients have not been assessed yet, justifying the necessity to conduct a clinical study in this field. c.
Keywords: COVID-19; Critically ill; ICU; Mortality; l-carnitine.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Jul 6;22(1):434. doi: 10.1186/s13063-021-05372-9. Trials. 2021. PMID: 34229742 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
The effects of L-carnitine supplementation on inflammatory factors, oxidative stress, and clinical outcomes in patients with sepsis admitted to the intensive care unit (ICU): study protocol for a double blind, randomized, placebo-controlled clinical trial.Trials. 2022 Feb 22;23(1):170. doi: 10.1186/s13063-022-06077-3. Trials. 2022. PMID: 35193654 Free PMC article.
-
The effects of l-Carnitine supplementation on inflammatory markers, clinical status, and 28 days mortality in critically ill patients: A double-blind, randomized, placebo-controlled trial.Clin Nutr ESPEN. 2022 Jun;49:61-67. doi: 10.1016/j.clnesp.2022.04.001. Epub 2022 Apr 9. Clin Nutr ESPEN. 2022. PMID: 35623869 Clinical Trial.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
Cited by
-
Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective.Life (Basel). 2025 Jan 16;15(1):113. doi: 10.3390/life15010113. Life (Basel). 2025. PMID: 39860053 Free PMC article. Review.
References
-
- Organization WH . vol. 25. 2020. (Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance). January 2020.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous