Host-Viral Interactions at the Maternal-Fetal Interface. What We Know and What We Need to Know
- PMID: 36742289
- PMCID: PMC9894500
- DOI: 10.3389/fviro.2022.833106
Host-Viral Interactions at the Maternal-Fetal Interface. What We Know and What We Need to Know
Abstract
In humans, the hemochorial placenta is a unique temporary organ that forms during pregnancy to support fetal development, gaseous exchange, delivery of nutrition, removal of waste products, and provides immune protection, while maintaining tolerance to the HLA-haploidentical fetus. In this review, we characterize decidual and placental immunity during maternal viral (co)-infection with HIV-1, human cytomegalovirus (HCMV), and Zika virus. We discuss placental immunology, clinical presentation, and epidemiology, before characterizing host susceptibility and cellular tropism, and how the three viruses gain access into specific placental target cells. We describe current knowledge on host-viral interactions with decidual and stromal human placental macrophages or Hofbauer cells, trophoblasts including extra villous trophoblasts, T cells, and decidual natural killer (dNK) cells. These clinically significant viral infections elicit both innate and adaptive immune responses to control replication. However, the three viruses either during mono- or co-infection (HIV-1 and HCMV) escape detection to initiate placental inflammation associated with viral transmission to the developing fetus. Aside from congenital or perinatal infection, other adverse pregnancy outcomes include preterm labor and spontaneous abortion. In addition, maternal HIV-1 and HCMV co-infection are associated with impaired fetal and infant immunity in postnatal life and poor clinical outcomes during childhood in exposed infants, even in the absence of vertical transmission of HIV-1. Given the rapidly expanding numbers of HIV-1-exposed uninfected infants and children globally, further research is urgently needed on neonatal immune programming during maternal mono-and co-infection. This review therefore includes sections on current knowledge gaps that may prompt future research directions. These gaps reflect an emerging but poorly characterized field. Their significance and potential investigation is underscored by the fact that although viral infections result in adverse consequences in both mother and developing fetus/newborn, antiviral and immunomodulatory therapies can improve clinical outcomes in the dyad.
Keywords: HIV-1; Hofbauer cell; Zika; cytotrophoblast; human cytomegalovirus; natural killer cell; placenta; vertical transmission.
Conflict of interest statement
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

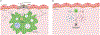



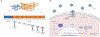
References
-
- Mossman HW. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology, Evolution, Phylogenetic Significance, Basic Functions, Research Opportunities. New Brunswick: Rutgers University Press; (1987).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
