Circulating levels and the bioactivity of miR-30b increase during pubertal progression in boys
- PMID: 36742409
- PMCID: PMC9893272
- DOI: 10.3389/fendo.2023.1120115
Circulating levels and the bioactivity of miR-30b increase during pubertal progression in boys
Abstract
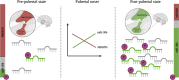
Background: Puberty marks the transition from childhood to adulthood and is initiated by activation of a pulsatile GnRH secretion from the hypothalamus. MKRN3 functions as a pre-pubertal break on the GnRH pulse generator and hypothalamic expression and circulating levels of MKRN3 decrease peri-pubertally. In rodents, microRNA miR-30b seems to directly target hypothalamic MKRN3 expression - and in boys, circulating levels of miR-30b-5p increase when puberty is pharmacologically induced. Similarly, miR-200b-3p and miR-155-5p have been suggested to inhibit expression of other proteins potentially involved in the regulation of GnRH secretion. Here we measure circulating levels of these three miRNAs as boys progress through puberty.
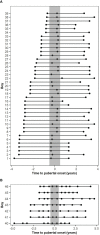
Materials and methods: Forty-six boys from the longitudinal part of the Copenhagen Puberty Study were included. All boys underwent successive clinical examinations including estimation of testis size by palpation. miR-30b-5p, miR-200b-3p, and miR-155-5p were measured in serum by RT-qPCR using a kit sensitive to the phosphorylation status of the miRNAs. Thirty-nine boys had miRNA levels measured in three consecutive samples (pre-, peri-, and post-pubertally) and seven boys had miR-30b-5p levels measured in ten consecutive samples during the pubertal transition.
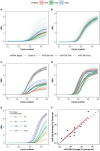
Results: When circulating levels of miR-30b-5p in pre- and peri-pubertal samples were compared with post-pubertal levels, we observed a significant increase of 2.3 and 2.2-fold (p-value<6.0×10-4), respectively, and a larger fraction of miR-30b-5p appeared to be phosphorylated post-pubertally indicating an increase in its bioactivity. We also observed a negative correlation between circulating levels of miR-30b-5p and MKRN3. The inter-individual variation in circulating miR-30b levels was substantial and we could not define a clinical threshold for miR-30b-5p suggestive of imminent puberty. Also, miR-155-5p showed significantly increasing levels from the peri- to the post-pubertal stage (p=3.0×10-3), whereas miR-200b-3p did not consistently increase.
Conclusion: Both circulating levels of miR-30b-5p and its bioactivity increase during the pubertal transition in boys supporting its role in the activation of the HPG axis at the onset of physiologically normal puberty.
Keywords: HPG-axis; MKRN3; biomarker; miR-30b; miRNA; puberty.
Copyright © 2023 Mørup, Stakaitis, Main, Golubickaite, Hagen, Juul and Almstrup.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kaprara A, Huhtaniemi IT. The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism (2018) 86:3–17. - PubMed
-
- Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: Minipuberty. Horm Res Paediatr (2014) 82:73–80. - PubMed
-
- Messina A, Langlet F, Chachlaki K, Roa J, Rasika S, Jouy N, et al. . A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci (2016) 19:835–44. - PubMed
-
- Ojeda SR, Lomniczi A. Puberty in 2013: Unravelling the mystery of puberty. Nat Rev Endocrinol (2014) 10:67–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

