Augmentation of IFN-γ by bone marrow derived immune cells in the presence of severe suppression of IFN-γ in gingivae induced by zoledronic acid and denosumab in Hu-BLT mice model of ONJ
- PMID: 36742414
- PMCID: PMC9895394
- DOI: 10.3389/fendo.2023.1111627
Augmentation of IFN-γ by bone marrow derived immune cells in the presence of severe suppression of IFN-γ in gingivae induced by zoledronic acid and denosumab in Hu-BLT mice model of ONJ
Abstract
Introduction: The potential mechanisms governing drug induced osteonecrosis of the jaw (ONJ) is not well understood, and is one of the objectives of this study. Thus, we tested the release of IFN-γ within different immune compartments including bone marrow and gingivae upon treatment with zoledronic acid (ZOL) and denosumab which are known to induce ONJ in susceptible individuals.
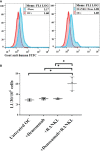
Methods: We used humanized-BLT mouse model for the in-vivo studies reported in this paper. To determine the effects of zoledronic acid and denosumab on IFN-γ secretion and NK cell-mediated cytotoxicity; peripheral blood, bone marrow, spleen and gingiva were obtained after the injection of ZOL and denosumab in mice.
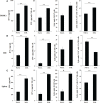
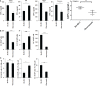
Results: Percentages of B cells are much higher in wild-type mice whereas the proportions of immune subsets in humans and reconstituted hu-BLT peripheral-blood are similar. Therefore, hu-BLT mice are preferable model to study human disease, in particular, immune-pathologies induced by ZOL and denosumab. Both agents resulted in a severe suppression of IFN-γ in the gingiva, whereas they heightened the release of IFN-γ and NK cell-mediated cytotoxicity by the BM-derived immune cells. ZOL increased the IFN-γ secretion by the spleen and peripheral blood immune cells, whereas denosumab decreased the release IFN-γ by these cells significantly.
Discussion: ZOL and denosumab may likely suppress IFN-γ secretion in gingiva through different mechanisms. In addition, to the suppression of IFN-γ secretion, denosumab mediated effect could in part be due to the decrease in the bone resorptive function of osteoclasts due to the induction of antibody dependent cellular cytotoxicity and lysis of osteoclasts, whereas ZOL is able to mediate cell death of osteoclasts directly. Suppression of IFN-gamma in gingiva is largely responsible for the inhibition of immune cell function, leading to dysregulated osteoblastic and osteoclastic activities. Restoration of IFN-gamma in the local microenvironment may result in establishment of homeostatic balance in the gingiva and prevention of osteonecrosis of jaw.
Keywords: IFN-γ; NK cells; antibody-dependent cellular cytotoxicity (ADCC); cytotoxicity; denosumab; humanized-BLT mice; osteonecrosis of the jaw; zoledronic acid.
Copyright © 2023 Kaur, Sun, Kanayama, Morinaga, Hokugo, Nishimura and Jewett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Dehari H, Tomihara K, Ueda M, Shimanishi M, Ono M, Sasaki T, et al. . [Clinical investigation of bisphosphonate-related osteonecrosis of the jaws]. Gan To Kagaku Ryoho (2009) 36(13):2587–92. - PubMed

