OSA 18 Questionnaire: Tool to Evaluate Quality of Life and Efficacy of Treatment Modalities in Pediatric Sleep Disordered Breathing Due to Adenotonsillar Hypertrophy
- PMID: 36742702
- PMCID: PMC9895508
- DOI: 10.1007/s12070-019-01757-0
OSA 18 Questionnaire: Tool to Evaluate Quality of Life and Efficacy of Treatment Modalities in Pediatric Sleep Disordered Breathing Due to Adenotonsillar Hypertrophy
Abstract
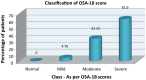
Aims to evaluate quality of life in paediatric SDB due to adenotonsillar hypertrophy and efficacy of treatment modalities (medical and surgical) by using OSA-18 questionnaire. Prospective study, conducted from April 2019 to June 2019, including 42 patients with clinical features suggestive of SDB due to adenotonsillar hypertrophy, in age group of 3-15 years. Nasopharyngoscopy was done to grade adenoid hypertrophy. OSA-18 QOL questionnaire was recorded in all patients and depending upon the severity of impact of QOL and grades of adenoid hypertrophy, patients were categorized into two groups. Group 1 received medical treatment and group 2 underwent adenotonsillectomy. Questionnaire was again recorded after 4 weeks. Pretreatment and post-treatment total mean and individual domain scores were compared. Paired t tests was used to evaluate results. Group 1 included 16 children with mild to moderate impact and received medical management. Pretreatment mean OSA-18 score of 70.31 was improved to 33.5. Group 2 enrolled 26 patients with severe impact, were subjected to adenotonsillectomy. Pretreatment and post-treatment mean score were 95.88 and 24.92 respectively. Both groups showed statistically significant improvement in all individual domains and total mean OSA-18 scores indicating improvement in QOL after treatment and efficacy of medical management for mild-moderate SDB and surgery for severe cases. OSA-18 questionnaire is self-administered and disease specific screening tool for early diagnosis and evaluation of QOL before and after treatment. It also helps to categorize patients for advocating appropriate treatment and to evaluate efficacy of treatment modalities.
Keywords: Adenotonsillectomy; Mometasone nasal spray; Montelukast; Obstructive sleep apnea; Quality of life OSA 18 questionnaire; Sleep disordered breathing.
© Association of Otolaryngologists of India 2019.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures
Similar articles
-
Can sleep questionnaires predict adenotonsillectomy outcome for children with sleep disordered breathing?Int J Pediatr Otorhinolaryngol. 2022 Feb;153:111001. doi: 10.1016/j.ijporl.2021.111001. Epub 2021 Dec 15. Int J Pediatr Otorhinolaryngol. 2022. PMID: 34952376
-
Long-term Impact of Adenotonsillectomy on the Quality of Life of Children with Sleep-disordered breathing.Int Arch Otorhinolaryngol. 2021 Jan;25(1):e123-e128. doi: 10.1055/s-0040-1709195. Epub 2020 May 4. Int Arch Otorhinolaryngol. 2021. PMID: 33542762 Free PMC article.
-
Post Surgical Outcomes in Paediatric Adenotonsillar Hypertrophy with Obstructive Sleep Apnea: Subjective and Objective Evaluation.Indian J Otolaryngol Head Neck Surg. 2023 Apr;75(Suppl 1):822-827. doi: 10.1007/s12070-022-03453-y. Epub 2022 Dec 31. Indian J Otolaryngol Head Neck Surg. 2023. PMID: 37206789 Free PMC article.
-
The efficacy and safety of montelukast in children with obstructive sleep apnea: a systematic review and meta-analysis.Sleep Med. 2021 Feb;78:193-201. doi: 10.1016/j.sleep.2020.11.009. Epub 2020 Nov 10. Sleep Med. 2021. PMID: 33465554
-
Anti-inflammatory medications for obstructive sleep apnoea in children.Cochrane Database Syst Rev. 2020 Jan 17;1(1):CD007074. doi: 10.1002/14651858.CD007074.pub3. Cochrane Database Syst Rev. 2020. PMID: 31978261 Free PMC article.
Cited by
-
Quality of life related to residual snoring after adenotonsillectomy: a pilot study.Sleep Sci. 2021 Jan-Mar;14(4):330-336. doi: 10.5935/1984-0063.20200093. Sleep Sci. 2021. PMID: 35087629 Free PMC article.
-
Quality of life of elementary school students with sleep-disordered breathing and allergic rhinitis: A population-based study in Thailand.PLoS One. 2024 Sep 11;19(9):e0310331. doi: 10.1371/journal.pone.0310331. eCollection 2024. PLoS One. 2024. PMID: 39259725 Free PMC article.
-
A narrative review on obstructive sleep apnoea syndrome in paediatric population.Front Neurol. 2024 Jul 5;15:1393272. doi: 10.3389/fneur.2024.1393272. eCollection 2024. Front Neurol. 2024. PMID: 39036631 Free PMC article. Review.
-
An Objective Study to Establish Incidence of True Obstructive Sleep Apnoea (OSA) in Sleep Disordered Breathing in the Paediatric Age Group and Assessment of Benefit of Surgery (Tonsillectomy and Adenoidectomy) in Non Responders to Medical Treatment in Mild OSA.Indian J Otolaryngol Head Neck Surg. 2024 Oct;76(5):4189-4199. doi: 10.1007/s12070-024-04813-6. Epub 2024 Jun 25. Indian J Otolaryngol Head Neck Surg. 2024. PMID: 39376431
References
-
- Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956–958. - PubMed
-
- Brockmann PE, Urschitz MS, Schlaud M, et al. Primary snoring in school children: prevalence and neurocognitive impairments. Sleep Breath. 2012;16(1):23–29. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous