Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases
- PMID: 36743024
- PMCID: PMC9893457
- DOI: 10.1021/acsomega.2c06098
Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases
Abstract
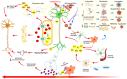
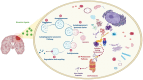
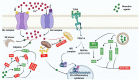
The brain is vulnerable to different types of stresses, particularly oxidative stress as a result of oxygen requirements/utilization in the body. Large amounts of unsaturated fatty acids present in the brain increase this vulnerability. Neurodegenerative diseases (NDDs) are brain disorders that are characterized by the gradual loss of specific neurons and are attributed to broad evidence of cell-level oxidative stress. The accurate characterization of neurological disorders relies on several parameters along with genetics and environmental risk factors, making therapies less efficient to fight NDDs. On the way to tackle oxidative damage and discover efficient and safe therapies, bioactives are at the edge of NDD science. Naturally occurring bioactive compounds such as polyphenols, carotenoids, essential fatty acids, phytosterols, essential oils, etc. are particularly of interest owing to their potent antioxidant and anti-inflammatory activities, and they offer lots of brain-health-promoting features. This Review focuses on probing the neuroefficacy and bioefficacy of bioactives and their role in supporting relatively low antioxidative and low regenerative capacities of the brain, neurogenesis, neuroprotection, and ameliorating/treating NDDs.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Testa G.; Staurenghi E.; Zerbinati C.; Gargiulo S.; Iuliano L.; Giaccone G.; Fantò F.; Poli G.; Leonarduzzi G.; Gamba P. Changes in Brain Oxysterols at Different Stages of Alzheimer’s Disease: Their Involvement in Neuroinflammation. Redox Biol. 2016, 10, 24–33. 10.1016/j.redox.2016.09.001. - DOI - PMC - PubMed
-
- Mahalakshmi K.; Parimalanandhini D.; Sangeetha R.; Livya Catherene M.; Beulaja M.; Thiagarajan R.; Arumugam M.; Janarthanan S.; Manikandan R. Influential Role of 7-Ketocholesterol in the Progression of Alzheimer’s Disease. Prostaglandins Other Lipid Mediat. 2021, 156, 106582.10.1016/j.prostaglandins.2021.106582. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

