Silver-mediated Room Temperature Reactions for the Synthesis of N-α-Ketoacyl Sulfoximines and N-α,β-Unsaturated Acyl Sulfoximines
- PMID: 36743040
- PMCID: PMC9893487
- DOI: 10.1021/acsomega.2c05894
Silver-mediated Room Temperature Reactions for the Synthesis of N-α-Ketoacyl Sulfoximines and N-α,β-Unsaturated Acyl Sulfoximines
Abstract
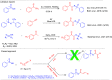
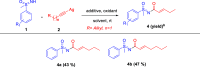
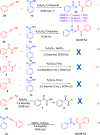
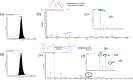
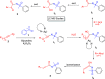
Here, we report a silver-mediated coupling of acetylenes with sulfoximines to synthesize N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines. The reactions are performed under an open atmosphere using the oxidant K2S2O8 and the ligand 2,2-bipyridyl. However, the fate of the product formation is controlled by the type of substrate used. The coupling between aryl acetylenes and sulfoximines afforded the N-α-ketoacylsulfoximines, while the alkyl acetylenes provided the N-α,β-unsaturated acyl sulfoximines. Controlled experiments reveal the differential reactivity patterns of substrates. The labeling 18O experiments showed that water is the source of the incoming oxygen atom for the keto group of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
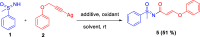
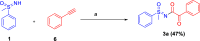
References
-
- Gais H.-J.; Babu G. S.; Günter M.; Das P. Asymmetric Synthesis of Cycloalkenyl and Alkenyloxiranes From Allylic Sulfoximines and Aldehydes and Application to Solid-Phase Synthesis. Eur. J. Org. Chem. 2004, 1464–1473. 10.1002/ejoc.200300726. - DOI
-
- Koep S.; Gais H.-J.; Raabe G. Asymmetric synthesis of unsaturated, fused bicyclic proline analogues through amino alkylation of cyclic bis(allylsulfoximine)titanium complexes and migratory cyclization of delta-amino alkenyl aminosulfoxonium salts. J. Am. Chem. Soc. 2003, 125, 13243–13251. 10.1021/ja030324y. - DOI - PubMed
LinkOut - more resources
Full Text Sources

