Clinical phase I/II trial of SVF therapy for cartilage regeneration: A cellular therapy with novel 3D MRI imaging for evaluating chondral defect of knee osteoarthritis
- PMID: 36743417
- PMCID: PMC9892457
- DOI: 10.3389/fcell.2023.1106279
Clinical phase I/II trial of SVF therapy for cartilage regeneration: A cellular therapy with novel 3D MRI imaging for evaluating chondral defect of knee osteoarthritis
Abstract
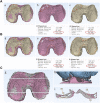
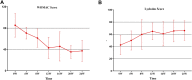
Background: The clinical applications of stromal vascular fraction (SVF) therapy for osteoarthritis (OA) have attracted academic and clinical attention. However, data of the effects of stromal vascular fraction therapy on regeneration of degenerated cartilage are limited in the literature. Meanwhile, there is a great need for a simple and non-invasive evaluation method to analyze the changes of joint cartilage qualitatively and quantitatively in clinical trials. This study entitled "stromal vascular fraction Therapy for Human Knee Osteoarthritis" was registered in ClinicalTrial.gov # NCT05019378. Materials and Methods: We designed and conducted a single center, open labeled clinical phase I/II study, and 6 osteoarthritis patients with both knee cartilage defect I-II were enrolled in this study. The two knees of each patient were randomly assigned to autologous stromal vascular fraction treatment group or non-treatment control group to evaluate the safety and therapeutic effect of stromal vascular fraction therapy for human knee osteoarthritis. We have also established a novel protocol to provide 3D MRI imaging for human knee cartilage enabling us to qualitatively and quantitatively evaluate cartilage degeneration and regeneration in this study. Results: The qualitative and quantitative evaluation of 3D Magnetic Resonance Imaging (MRI) imaging of knee cartilage demonstrated that the stromal vascular fraction therapy reduced the cartilage defects; and significant increase of cartilage value both in defect cartilage area and whole cartilage area of treated group and significant increase of thickness and area of both femoral and tibia cartilage in vertical sections of the stromal vascular fraction treated Group at 12 and 24 W post treatment in cartilage defect I-II osteoarthritis patients. Conclusion: This clinical phase I/II study indicated that stromal vascular fraction therapy is a safe clinical procedure and provided evidence that the stromal vascular fraction therapy significantly facilitated cartilage regeneration, opening the opportunity to a phase III trial investigating authentic efficacy of the procedure. This study is the first qualitative and quantitative evaluation of the efficacy of autologous stromal vascular fraction cellular therapy on cartilage regeneration. Through early and definite diagnosis of knee osteoarthritis patients, and providing safe and efficient therapy to facilitate cartilage regeneration, we will be able to control or reverse cartilage degeneration and completely change the epidemiology of osteoarthritis worldwide.
Keywords: 3D MRI imaging; cartilage regeneration; clinical trial; osteoarthritis; stromal vascular fraction (SVF).
Copyright © 2023 Ren, Chang, Liu, Xiao, Xu, Li, Li, Ruan, Bao, Lin, Zhou, Liao, Pan, Xu, Tian, Cai and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The Effect of Autologous Adipose-Derived Stromal Vascular Fractions on Cartilage Regeneration Was Quantitatively Evaluated Based on the 3D-FS-SPGR Sequence: A Clinical Trial Study.Biomed Res Int. 2022 Jan 25;2022:2777568. doi: 10.1155/2022/2777568. eCollection 2022. Biomed Res Int. 2022. PMID: 35118156 Free PMC article. Clinical Trial.
-
Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: a double-blind randomized self-controlled trial.Int Orthop. 2019 May;43(5):1123-1134. doi: 10.1007/s00264-018-4099-0. Epub 2018 Aug 14. Int Orthop. 2019. PMID: 30109404 Clinical Trial.
-
Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering.Regen Eng Transl Med. 2022 Jun;8(2):210-224. doi: 10.1007/s40883-021-00226-x. Epub 2021 Aug 11. Regen Eng Transl Med. 2022. PMID: 35958164 Free PMC article.
-
Autologous Adipose-Derived Tissue Stromal Vascular Fraction (AD-tSVF) for Knee Osteoarthritis.Int J Mol Sci. 2022 Nov 4;23(21):13517. doi: 10.3390/ijms232113517. Int J Mol Sci. 2022. PMID: 36362308 Free PMC article. Review.
-
Autologous and Allogenic Utilization of Stromal Vascular Fraction and Decellularized Extracellular Matrices in Orthopedic Surgery: A Scoping Review.Arch Bone Jt Surg. 2022 Oct;10(10):827-832. doi: 10.22038/ABJS.2022.59635.2943. Arch Bone Jt Surg. 2022. PMID: 36452418 Free PMC article.
Cited by
-
Stem cell injections for osteoarthritis of the knee.Cochrane Database Syst Rev. 2025 Apr 2;4(4):CD013342. doi: 10.1002/14651858.CD013342.pub2. Cochrane Database Syst Rev. 2025. PMID: 40169165
-
Rapid-acting pain relief in knee osteoarthritis: autologous-cultured adipose-derived mesenchymal stem cells outperform stromal vascular fraction: a systematic review and meta-analysis.Stem Cell Res Ther. 2024 Nov 21;15(1):446. doi: 10.1186/s13287-024-04034-2. Stem Cell Res Ther. 2024. PMID: 39568086 Free PMC article.
-
Injection of Autologous Adipose Stromal Vascular Fraction in Combination with Autologous Conditioned Plasma for the Treatment of Advanced Knee Osteoarthritis Significantly Improves Clinical Symptoms.J Clin Med. 2024 May 22;13(11):3031. doi: 10.3390/jcm13113031. J Clin Med. 2024. PMID: 38892743 Free PMC article.
-
Regenerative Cartilage Treatment for Focal Chondral Defects in the Knee: Focus on Marrow-Stimulating and Cell-Based Scaffold Approaches.Cells. 2025 Aug 7;14(15):1217. doi: 10.3390/cells14151217. Cells. 2025. PMID: 40801648 Free PMC article. Review.
-
Current Status of Stromal Vascular Fraction from Adipose Tissue in the Clinical Application for Osteoarthritis Treatment.Tissue Eng Regen Med. 2025 Aug;22(6):747-754. doi: 10.1007/s13770-025-00722-z. Epub 2025 Jun 6. Tissue Eng Regen Med. 2025. PMID: 40478499 Review.
References
-
- Bourin P., Bunnell B. A., Casteilla L., Dominici M., Katz A. J., March K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT). Cytotherapy 15, 641–648. 10.1016/j.jcyt.2013.02.006 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

