Computational design of custom therapeutic cells to correct failing human cardiomyocytes
- PMID: 36743445
- PMCID: PMC9894098
- DOI: 10.3389/fsysb.2023.1102467
Computational design of custom therapeutic cells to correct failing human cardiomyocytes
Abstract
Background: Myocardial delivery of non-excitable cells-namely human mesenchymal stem cells (hMSCs) and c-kit+ cardiac interstitial cells (hCICs)-remains a promising approach for treating the failing heart. Recent empirical studies attempt to improve such therapies by genetically engineering cells to express specific ion channels, or by creating hybrid cells with combined channel expression. This study uses a computational modeling approach to test the hypothesis that custom hypothetical cells can be rationally designed to restore a healthy phenotype when coupled to human heart failure (HF) cardiomyocytes.
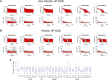
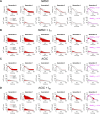
Methods: Candidate custom cells were simulated with a combination of ion channels from non-excitable cells and healthy human cardiomyocytes (hCMs). Using a genetic algorithm-based optimization approach, candidate cells were accepted if a root mean square error (RMSE) of less than 50% relative to healthy hCM was achieved for both action potential and calcium transient waveforms for the cell-treated HF cardiomyocyte, normalized to the untreated HF cardiomyocyte.
Results: Custom cells expressing only non-excitable ion channels were inadequate to restore a healthy cardiac phenotype when coupled to either fibrotic or non-fibrotic HF cardiomyocytes. In contrast, custom cells also expressing cardiac ion channels led to acceptable restoration of a healthy cardiomyocyte phenotype when coupled to fibrotic, but not non-fibrotic, HF cardiomyocytes. Incorporating the cardiomyocyte inward rectifier K+ channel was critical to accomplishing this phenotypic rescue while also improving single-cell action potential metrics associated with arrhythmias, namely resting membrane potential and action potential duration. The computational approach also provided insight into the rescue mechanisms, whereby heterocellular coupling enhanced cardiomyocyte L-type calcium current and promoted calcium-induced calcium release. Finally, as a therapeutically translatable strategy, we simulated delivery of hMSCs and hCICs genetically engineered to express the cardiomyocyte inward rectifier K+ channel, which decreased action potential and calcium transient RMSEs by at least 24% relative to control hMSCs and hCICs, with more favorable single-cell arrhythmia metrics.
Conclusion: Computational modeling facilitates exploration of customizable engineered cell therapies. Optimized cells expressing cardiac ion channels restored healthy action potential and calcium handling phenotypes in fibrotic HF cardiomyocytes and improved single-cell arrhythmia metrics, warranting further experimental validation studies of the proposed custom therapeutic cells.
Keywords: action potential; calcium transient; cardiac electrophysiology; cell therapy; computational modeling; heart failure; heterocellular coupling.
Conflict of interest statement
Conflict of interest KDC discloses his role as scientific co-founder and Chief Scientific Officer of Novoheart International Ltd. Novoheart did not play any role in the design or conduct of this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bolli R., Mitrani R. D., Hare J. M., Pepine C. J., Perin E. C., Willerson J. T., et al. (2021). A phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur. J. Heart Fail 23, 661–674. 10.1002/ejhf.2178 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

