ROS-mediated plasmodesmal regulation requires a network of an Arabidopsis receptor-like kinase, calmodulin-like proteins, and callose synthases
- PMID: 36743578
- PMCID: PMC9893415
- DOI: 10.3389/fpls.2022.1107224
ROS-mediated plasmodesmal regulation requires a network of an Arabidopsis receptor-like kinase, calmodulin-like proteins, and callose synthases
Abstract
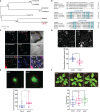
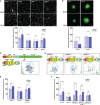
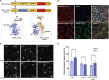
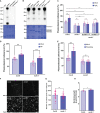
Plasmodesmata (PD) play a critical role in symplasmic communication, coordinating plant activities related to growth & development, and environmental stress responses. Most developmental and environmental stress signals induce reactive oxygen species (ROS)-mediated signaling in the apoplast that causes PD closure by callose deposition. Although the apoplastic ROS signals are primarily perceived at the plasma membrane (PM) by receptor-like kinases (RLKs), such components involved in PD regulation are not yet known. Here, we show that an Arabidopsis NOVEL CYS-RICH RECEPTOR KINASE (NCRK), a PD-localized protein, is required for plasmodesmal callose deposition in response to ROS stress. We identified the involvement of NCRK in callose accumulation at PD channels in either basal level or ROS-dependent manner. Loss-of-function mutant (ncrk) of NCRK induces impaired callose accumulation at the PD under the ROS stress resembling a phenotype of the PD-regulating GLUCAN SYNTHASE-LIKE 4 (gsl4) knock-out plant. The overexpression of transgenic NCRK can complement the callose and the PD permeability phenotypes of ncrk mutants but not kinase-inactive NCRK variants or Cys-mutant NCRK, in which Cys residues were mutated in Cys-rich repeat ectodomain. Interestingly, NCRK mediates plasmodesmal permeability in mechanical injury-mediated signaling pathways regulated by GSL4. Furthermore, we show that NCRK interacts with calmodulin-like protein 41 (CML41) and GSL4 in response to ROS stress. Altogether, our data indicate that NCRK functions as an upstream regulator of PD callose accumulation in response to ROS-mediated stress signaling pathways.
Keywords: ROS perception; abiotic and biotic stress; callose; plasmodesmata; receptor-like kinase (RLK).
Copyright © 2023 Vu, Hyun, Bahk, Jo, Kumar, Thiruppathi, Iswanto, Chung, Shelake and Kim.
Conflict of interest statement
J-YK is a founder and CEO of Nulla Bio Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Localization of Arabidopsis Glucan Synthase-Like 5, 8, and 12 to plasmodesmata and the GSL8-dependent role of PDLP5 in regulating plasmodesmal permeability.Plant Signal Behav. 2023 Dec 31;18(1):2164670. doi: 10.1080/15592324.2022.2164670. Plant Signal Behav. 2023. PMID: 36645916 Free PMC article.
-
Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress.Nat Plants. 2016 Apr 11;2(5):16034. doi: 10.1038/nplants.2016.34. Nat Plants. 2016. PMID: 27243643
-
Plasma Membrane-Associated Receptor-like Kinases Relocalize to Plasmodesmata in Response to Osmotic Stress.Plant Physiol. 2019 Sep;181(1):142-160. doi: 10.1104/pp.19.00473. Epub 2019 Jul 12. Plant Physiol. 2019. PMID: 31300470 Free PMC article.
-
Biology of callose (β-1,3-glucan) turnover at plasmodesmata.Protoplasma. 2011 Jan;248(1):117-30. doi: 10.1007/s00709-010-0247-0. Epub 2010 Nov 30. Protoplasma. 2011. PMID: 21116665 Free PMC article. Review.
-
Callose balancing at plasmodesmata.J Exp Bot. 2018 Nov 26;69(22):5325-5339. doi: 10.1093/jxb/ery317. J Exp Bot. 2018. PMID: 30165704 Review.
Cited by
-
Plasmodesmata Function and Callose Deposition in Plant Disease Defense.Plants (Basel). 2024 Aug 13;13(16):2242. doi: 10.3390/plants13162242. Plants (Basel). 2024. PMID: 39204678 Free PMC article. Review.
-
Role of Tyrosine Phosphorylation in PEP1 Receptor 1(PEPR1) in Arabidopsis thaliana.Plants (Basel). 2025 May 19;14(10):1515. doi: 10.3390/plants14101515. Plants (Basel). 2025. PMID: 40431080 Free PMC article.
-
Glucosinolates can act as signals to modulate intercellular trafficking via plasmodesmata.New Phytol. 2025 May;246(3):1163-1182. doi: 10.1111/nph.70032. Epub 2025 Mar 17. New Phytol. 2025. PMID: 40095529
-
Advances in understanding the graft healing mechanism: a review of factors and regulatory pathways.Hortic Res. 2024 Jun 20;11(8):uhae175. doi: 10.1093/hr/uhae175. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39108577 Free PMC article.
-
The multifarious role of callose and callose synthase in plant development and environment interactions.Front Plant Sci. 2023 May 31;14:1183402. doi: 10.3389/fpls.2023.1183402. eCollection 2023. Front Plant Sci. 2023. PMID: 37324665 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

