Cell-type specific distribution and activation of type I IFN pathway molecules at the placental maternal-fetal interface in response to COVID-19 infection
- PMID: 36743911
- PMCID: PMC9895786
- DOI: 10.3389/fendo.2022.951388
Cell-type specific distribution and activation of type I IFN pathway molecules at the placental maternal-fetal interface in response to COVID-19 infection
Abstract
Background and objective: COVID-19 infection in pregnancy significantly increases risks of adverse pregnancy outcomes. However, little is known how the innate immunity at the placental maternal-fetal interface responds to COVID-19 infection. Type I IFN cytokines are recognized as a key component of the innate immune response against viral infection. In this study, we specifically evaluated expression of IFN antiviral signaling molecules in placentas from women infected with COVID-19 during pregnancy.
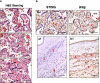
Methods: Expression of IFN activation signaling pathway molecules, including cyclic GMP-AMP synthase (cGAS), stimulator of interferon genes (STING), interferon regulatory factor 3 (IRF3), Toll-like receptor 7 (TLR7), mitochondrial antiviral-signaling protein (MAVS), and IFNβ were determined in formalin-fixed paraffin embedded (FFPE) placental tissue sections (villous and fetal membrane) by immunostaining. A total of 20 placentas were examined, 12 from COVID-19 patients and 8 from non-COVID-19 controls. Patient demographics, clinical data, and placental pathology report were acquired via EPIC medical record review.
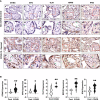
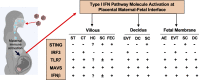
Results: Except BMI and placental weight, there was no statistical difference between COVID and non-COVID groups in maternal age, gestational age at delivery, gravity/parity, delivery mode, and newborn gender and weight. In COVID-exposed group, the main pathological characteristics in the placental disc are maternal and fetal vascular malperfusion and chronic inflammation. Compared to non-COVID controls, expression of IFN activation pathway molecules were all upregulated with distinct cell-type specific distribution in COVID-exposed placentas: STING in villous and decidual stromal cells; IRF3 in cytotrophoblasts (CTs) and extra-villous trophoblasts (EVTs); and TLR7 and MAVS in syncytiotrophoblasts (STs), CTs, and EVTs. Upregulation of STING, MAVS and TLR7 was also seen in fetal endothelial cells.
Conclusions: STING, IRF3, TLR7, and MAVS are key viral sensing molecules that regulate type I IFN production. Type I IFNs are potent antiviral cytokines to impair and eradicate viral replication in infected cells. The finding of cell-type specific distribution and activation of these innate antiviral molecules at the placental maternal-fetal interface provide plausible evidence that type I IFN pathway molecules may play critical roles against SARS-CoV-2 infection in the placenta. Our findings also suggest that placental maternal-fetal interface has a well-defined antiviral defense system to protect the developing fetus from SARS-CoV-2 infection.
Keywords: COVID-19; IRF3; MAVS; STING; TLR7; placental maternal-fetal interface; pregnancy; type I IFNs.
Copyright © 2023 Wang, Gu, Lewis, Gu, Brown, Lachute, Hankins, Scott, Busada, Cooper, McCathran and Barrilleaux.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Diminished antiviral innate immune gene expression in the placenta following a maternal SARS-CoV-2 infection.Am J Obstet Gynecol. 2023 Apr;228(4):463.e1-463.e20. doi: 10.1016/j.ajog.2022.09.023. Epub 2022 Sep 17. Am J Obstet Gynecol. 2023. PMID: 36126729 Free PMC article.
-
Toll-like receptor 7 (TLR7)-mediated antiviral response protects mice from lethal SARS-CoV-2 infection.J Virol. 2025 May 20;99(5):e0166824. doi: 10.1128/jvi.01668-24. Epub 2025 Mar 31. J Virol. 2025. PMID: 40162785 Free PMC article.
-
SARS-CoV-2 NSP7 inhibits type I and III IFN production by targeting the RIG-I/MDA5, TRIF, and STING signaling pathways.J Med Virol. 2023 Mar;95(3):e28561. doi: 10.1002/jmv.28561. J Med Virol. 2023. PMID: 36755358
-
SARS-CoV-2, HIV, and HPV: Convergent evolution of selective regulation of cGAS-STING signaling.J Med Virol. 2023 Jan;95(1):e28220. doi: 10.1002/jmv.28220. Epub 2022 Oct 26. J Med Virol. 2023. PMID: 36229923 Free PMC article. Review.
-
How Dengue Virus Circumvents Innate Immunity.Front Immunol. 2018 Dec 4;9:2860. doi: 10.3389/fimmu.2018.02860. eCollection 2018. Front Immunol. 2018. PMID: 30564245 Free PMC article. Review.
Cited by
-
Vitamin D Supplementation in Neonatal and Infant MIS-C Following COVID-19 Infection.Int J Mol Sci. 2024 Mar 27;25(7):3712. doi: 10.3390/ijms25073712. Int J Mol Sci. 2024. PMID: 38612523 Free PMC article. Review.
-
Maternal Immune Activation: Implications for Congenital Heart Defects.Clin Rev Allergy Immunol. 2025 Apr 2;68(1):36. doi: 10.1007/s12016-025-09049-y. Clin Rev Allergy Immunol. 2025. PMID: 40175706 Review.
-
Lactoferrin Supplementation in Preventing and Protecting from SARS-CoV-2 Infection: Is There Any Role in General and Special Populations? An Updated Review of Literature.Int J Mol Sci. 2024 Sep 24;25(19):10248. doi: 10.3390/ijms251910248. Int J Mol Sci. 2024. PMID: 39408576 Free PMC article. Review.
References
-
- Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. . Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr (2021) 175(8):817–26. doi: 10.1001/jamapediatrics.2021.1050 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

