Real world effectiveness of subcutaneous semaglutide in type 2 diabetes: A retrospective, cohort study (Sema-MiDiab01)
- PMID: 36743930
- PMCID: PMC9889982
- DOI: 10.3389/fendo.2022.1099451
Real world effectiveness of subcutaneous semaglutide in type 2 diabetes: A retrospective, cohort study (Sema-MiDiab01)
Abstract
Introduction: Aim of the present study was to evaluate the real-world impact of once-weekly (OW) subcutaneous semaglutide on different end-points indicative of metabolic control, cardiovascular risk factors, and beta-cell function in type 2 diabetes (T2D).
Methods: This was a retrospective, observational study conducted in 5 diabetes clinics in Italy. Changes in HbA1c, fasting blood glucose (FBG), body weight, blood pressure, lipid profile, renal function, and beta-cell function (HOMA-B) during 12 months were evaluated.
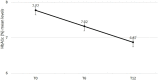
Results: Overall, 594 patients (97% GLP-1RA naïve) were identified (mean age 63.9 ± 9.5 years, 58.7% men, diabetes duration 11.4 ± 8.0 years). After 6 months of treatment with OW semaglutide, HbA1c levels were reduced by 0.90%, FBG by 26 mg/dl, and body weight by 3.43 kg. Systolic blood pressure, total and LDL-cholesterol significantly improved. Benefits were sustained at 12 months. Renal safety was documented. HOMA-B increased from 40.2% to 57.8% after 6 months (p<0.0001).
Discussion: The study highlighted benefits of semaglutide on metabolic control, multiple CV risk factors, and renal safety in the real-world. Semaglutide seems to be an advisable option for preservation of β-cell function and early evidence suggests it might have a role in modifying insulin resistance (HOMA-IR), the pathogenetic basis of prediabetes and T2D.
Keywords: HbA1c; beta-cell function; cardiovascular risk factors; effectiveness; insulin resistance; semaglutide; type 2 diabetes.
Copyright © 2023 Berra, Rossi, Mirani, Ceccarelli Ceccarelli, Romano, Sassi, Peretti, Favacchio, Pastore, Folini, Graziano, Lunati, Solerte and Fiorina.
Conflict of interest statement
MR has received funding for research from NovoNordisk, Sanofi, Alfasigma, Artsana, AstraZeneca, Johnson&Johnson, Medtronic, Shionogi, SOBI, Meteda and Theras. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- SID-AMD standard italiani per la cura del diabete mellito (2018). Available at: https://aemmedi.it/wp-content/uploads/2009/06/AMD-Standard-unico1.pdf.