Cholesterol metabolism in the regulation of inflammatory responses
- PMID: 36744258
- PMCID: PMC9895399
- DOI: 10.3389/fphar.2023.1121819
Cholesterol metabolism in the regulation of inflammatory responses
Abstract
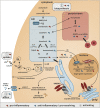
The importance of biologically active lipid mediators, such as prostanoids, leukotrienes, and specialized pro-resolving mediators, in the regulation of inflammation is well established. While the relevance of cholesterol in the context of atherosclerosis is also widely accepted, the role of cholesterol and its biosynthetic precursors on inflammatory processes is less comprehensively described. In the present mini-review, we summarize the current understanding of the inflammation-regulatory properties of cholesterol and relevant biosynthetic intermediates taking into account the implications of different subcellular distributions. Finally, we discuss the inflammation-regulatory effect of cholesterol homeostasis in the context of SARS-CoV-2 infections.
Keywords: COVID-19; SARS-CoV-2; cholesterol; immunometabolism; inflammation.
Copyright © 2023 Bauer, Brüne and Schmid.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

